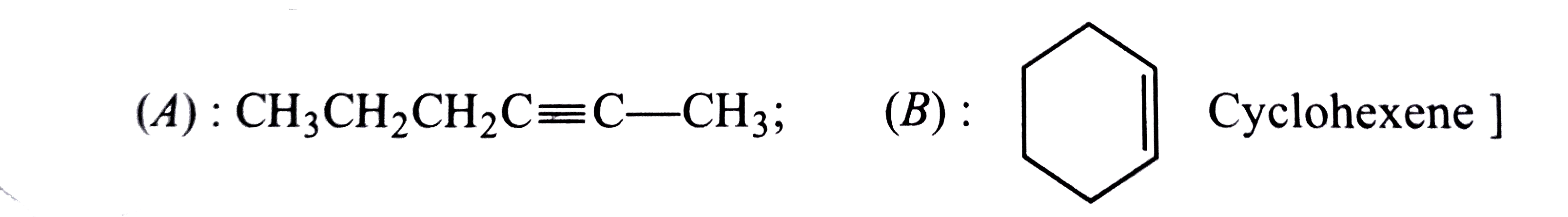Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Objective questions|498 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Integer|4 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
OP TANDON|Exercise Problem based on structure and properties|26 VideosBASIC PRINCIPLES OF ORGANIC COMPOUNDS (MECHANISM OF ORGANIC REACTIONS)
OP TANDON|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|4 VideosCHEMICAL KINETICS
OP TANDON|Exercise LINKED COMPRESHENSION TYPE QUESTIONS ( SECTION-VI)|4 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-CARBOXYLIC ACIDS AND THEIR DERIVATIVES-Brain storming problems
- Two isomeric compounds(A) and (B) have molecular formula C(6)H(10). O...
Text Solution
|
- Complete the following reactons
Text Solution
|
- Complete the following reactions:
Text Solution
|
- Arrange the following in decreasing order of acid catalysed estrificat...
Text Solution
|
- Arrange the following esters in the decreasing order of alkaline hydro...
Text Solution
|
- Arrange the following in decreasing order of acidic character:
Text Solution
|
- Complete the following reactions:
Text Solution
|
- Complete the following chain of reactions:
Text Solution
|
- Identify (A) to (F) in the following series of reactions:
Text Solution
|
- What are (A) and (B) in the following reactions ?
Text Solution
|
- Complete the following reactions
Text Solution
|
- How can you achieve the following interconversions ?
Text Solution
|
- Give the star mark to the hydrogen which is most acidic in the followi...
Text Solution
|
- What happens when malonic acid is heated with urea in presence of POCl...
Text Solution
|
- Complete the following reactions
Text Solution
|
- Complete the following reactions:
Text Solution
|
- What happens when benzene undergoes Friedel-Crafts acylation with succ...
Text Solution
|
- Complete the following Claisen condensation products. Indicate whether...
Text Solution
|
- Formation of napthalene takes place in the following steps. What are (...
Text Solution
|
- What are (X) and (Y) in the following reaction? Give the mechanism of ...
Text Solution
|