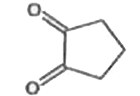
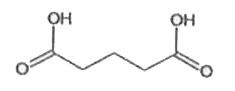
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise MORE THAN ONE CORRECT CHOICES|24 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise COMPREHENSION TYPE|18 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise Section (I) [Jee mains]: With Single Correct Choice|100 VideosGASEOUS STATE
FIITJEE|Exercise MATCHING LIST TYPE QUESTION|1 VideosHYDROCARBONS
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTION|9 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-GENERAL ORGANIC CHEMISTRY-Section (II) [Jee mains]: With Single Correct Choice
- The product is
Text Solution
|
- Which of the following will most readily dehydrated in acidic conditio...
Text Solution
|
- Oxidation product of 1,2-cyclopentane-diol with HlO(4) is
Text Solution
|
- X will be
Text Solution
|
- . Product X is
Text Solution
|
- Which among the following compounds are oxidised by NalO(4) Selec...
Text Solution
|
- Most probable structure of P is
Text Solution
|
- (A) is
Text Solution
|
- Consider the following two reactions : 1. The rate of reaction wi...
Text Solution
|
- Here x is
Text Solution
|
- In the reaction sequence (X) will be
Text Solution
|
- Product will be
Text Solution
|
- The reduction can be best carried out by
Text Solution
|
- A is
Text Solution
|
- Major product is
Text Solution
|
- What is the major product in the reaction?
Text Solution
|
- Compound (A) C(5)H(10)O forms a phenyl hydrogen and gives negative Tol...
Text Solution
|
- 2-methyl-2-phenyl propanamide when reacted with bromine and NaOH forms...
Text Solution
|
- Potassium pthalimide on reaction wiith (A) followed by hydrolysis form...
Text Solution
|
- Which of the following will give more enol contents ?
Text Solution
|