A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
KCET PREVIOUS YEAR PAPERS-KARNATAKA CET 2009-CHEMISTRY
- For the reaction H(2)O((i))hArrH(2)O((g)) at 373 K and 1 atmospheric p...
Text Solution
|
- A compound A(x)B(y) crystallises on a fcc lattice in which A occupies ...
Text Solution
|
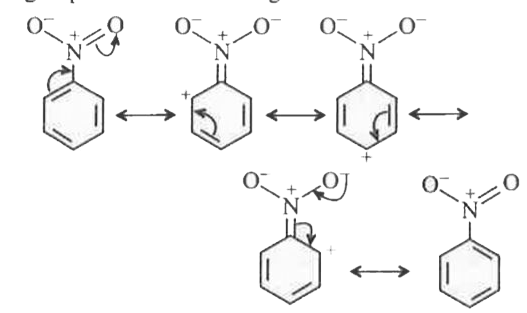
- In electrophilic aromatic substitution reaction, the nitro group is me...
Text Solution
|
- CH(3)COOHoverset(LiAlH(4))(to)X underset(300^(@)C)overset(Cu)(to) Y un...
Text Solution
|
- The best method for the conversion of an alcohol into an alkyl chlorid...
Text Solution
|
- The electrophile involved in the sulphonation of benzene is :
Text Solution
|
- The carbon-carbon bond length in benzene is :
Text Solution
|
- The compound which is not formed during the dry distillation of a mixt...
Text Solution
|
- An organic compound X is oxidised by using acidified K(2)Cr(2)O(7). Th...
Text Solution
|
- The reaction involved in the oil of Winter Green test is: salicylic ac...
Text Solution
|
- The compound which forms acetaldehyde when heated with dilute NaOH is
Text Solution
|
- Arrange the following in the increasing order of their basic strengths...
Text Solution
|
- The one which has least iodine value is
Text Solution
|
- A diabetic person carries a packet of glucose with him always, because
Text Solution
|
- There are 20 naturally occuring amino acids. The maximum number of tri...
Text Solution
|
- Cooking is fast in a pressure cooker because :
Text Solution
|
- The ore that is concentrated by froth floatation process is
Text Solution
|
- The correct set of four quantum numbers for outermost electron of pota...
Text Solution
|
- A body of mass x g is moving with a velocity of 100m/s. It de Broglie ...
Text Solution
|
- The correct order of ionisation enthalpy of C, N, O, F is
Text Solution
|