Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-Set II
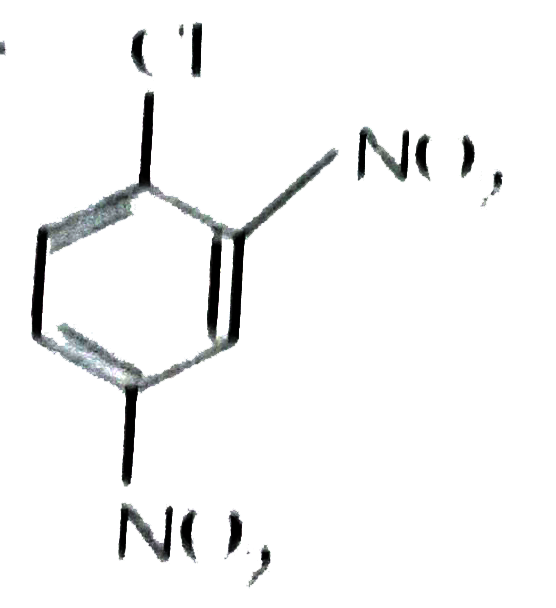
- Write the structure of 2.4-dinitrochlorobenzene.
Text Solution
|
- Write IUPAC name of the following compound : CH(3)NHCH (CH(3))(2)
Text Solution
|
- Drawn the structure of the following: (i) H(3)PO(2) (ii) XeF(4)
Text Solution
|
- Define the following terms: (i) Ideal solution (ii) Molarity (M)
Text Solution
|
- Complete the following reaction: (i) Cl(2) + H(2)O rarr (ii) XeF(6...
Text Solution
|
- What happens when: (i) conc. H(2)SO(4) is added to Cu ? (ii) SO(3)...
Text Solution
|
- Write the reactions involved in the following: (i) Hell-Volhard Zeli...
Text Solution
|
- Write the principles of the following methods: (i) Vapour phase refi...
Text Solution
|
- Define the following (i) Cationic detergents (ii) Narrow spectrum ...
Text Solution
|
- Write the structures of the monomers used for getting the following po...
Text Solution
|