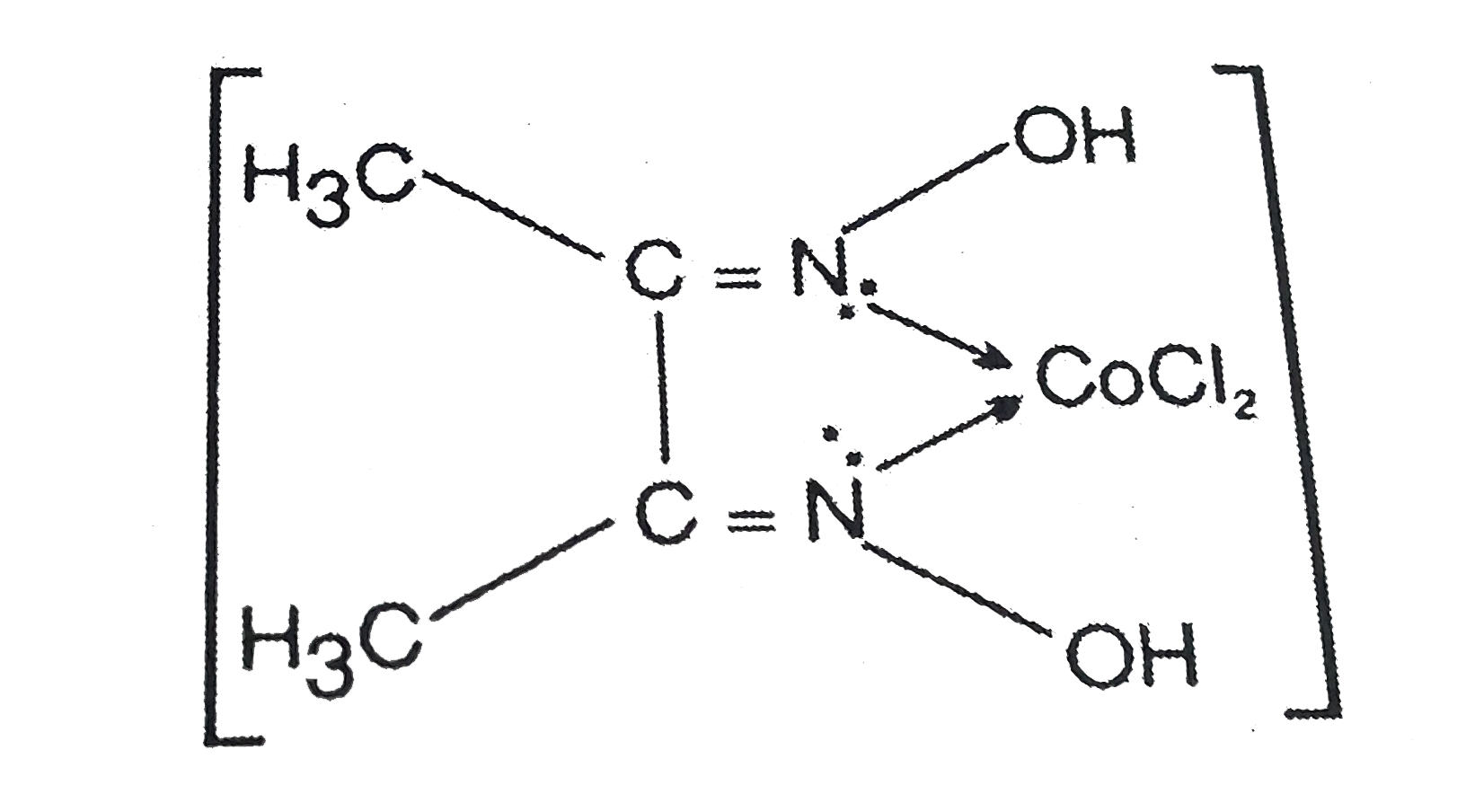
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-III Match the column|1 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-III Single and double value integer type|11 VideosCOORDINATION COMPOUNDS
RESONANCE|Exercise Additional Problem for Self Practice (APSP) Part-III Subjected Question|7 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE|Exercise INORGANIC CHEMISTRY(d & f- Block Elments)|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-COORDINATION COMPOUNDS-Additional Problem for Self Practice (APSP) Part-III Only one option correct type
- The correct IUPAC name of the complex is :
Text Solution
|
- A co-ordination complex has the formula PtCl(4).2KCl. Electrical condu...
Text Solution
|
- Which of the following complexes produces three moles of silver chlori...
Text Solution
|
- The number of chloride ions which would be precipated when one mole of...
Text Solution
|
- A coordination compound of cobalt has the molecular formula cotaining ...
Text Solution
|
- From the stability constant (hypothetical vlues ) , given below, predi...
Text Solution
|
- In Ziesses salt C=C bond length is : "Note":{{:(C-C"bond length in ...
Text Solution
|
- Which is not a pi-bonded complex ?
Text Solution
|
- What is wrong about the compound K[Pt(eta^(2)-C(2)H(4))Cl(3)] ?
Text Solution
|
- Which of the following are bidenate monoanion ligands ? (a) Dimethyl...
Text Solution
|
- Diethylenetriamine is :
Text Solution
|
- In K(4)[Fe(CN)(6)],Fe is in the form of
Text Solution
|
- Complex ion [FeN(3)(O(2))(SCN)(4)]^(4-) is named as : (coordination nu...
Text Solution
|
- The IUPAC name for K(2)[Cr(CN)(2)O(2)(O)(2)NH(3)] is:
Text Solution
|
- Consider the following statements: According the Werner's theory. ...
Text Solution
|
- Which of the following is correct for both the following coordination ...
Text Solution
|
- In the complexes [SbF(5)]^(2-),sp^(3)d hybridisation is present. Geome...
Text Solution
|
- Crystal field stabilization energy for high spin d^4 octahedral comple...
Text Solution
|
- underset("Brown")([(NH(3))(5)Co-O-O-Co(NH(3))(5)]^(4+)) underset("oxid...
Text Solution
|
- Which one of the following will be able to show cis-trans isomerism ?
Text Solution
|