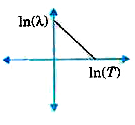
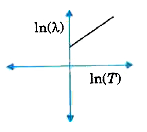
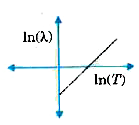
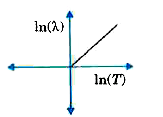
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
DUAL NATURE OF RADIATION AND MATTER
MODERN PUBLICATION|Exercise COMPETITION FILE (OBJECTIVE TYPE QUESTIONS ( B - Multiple Choice Questions) (AIPMT/NEET & Other State Boards for Medical Entrance))|26 VideosDUAL NATURE OF RADIATION AND MATTER
MODERN PUBLICATION|Exercise COMPETITION FILE (OBJECTIVE TYPE QUESTIONS ( B - Multiple Choice Questions) (JEE (Main) & Other State Boards for Engineering entrance))|18 VideosDUAL NATURE OF RADIATION AND MATTER
MODERN PUBLICATION|Exercise Revision Exercises (Numerical Problems)|20 VideosCURRENT ELECTRICITY
MODERN PUBLICATION|Exercise Chapter Practice Test|15 VideosELECTRIC CHARGES AND FIELDS
MODERN PUBLICATION|Exercise Chapter Practice Test|15 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-DUAL NATURE OF RADIATION AND MATTER -COMPETITION FILE (OBJECTIVE TYPE QUESTIONS ( A - Multiple Choice Questions))
- The graph showing the relation between de Broglie wavelength lamda of ...
Text Solution
|
- Photons of energy 5 eV and incident on a cathode made of a photosensit...
Text Solution
|
- The wavelength of light which is incident on a metal surface charge f...
Text Solution
|
- Which one of the following is not correct ?
Text Solution
|
- A metal is heated up to a certain temperature, the work function chang...
Text Solution
|
- Light of frequency v0 strikes on a metal surface and electrons are ej...
Text Solution
|
- Neglecting the mass variation with velocity , the ratio of the wavelen...
Text Solution
|
- A particle of mass 25 kg at rest decay into two practicals of messas ...
Text Solution
|
- Electron and photon are having equal wavelengths but difference masses...
Text Solution
|
- The following graph shows the variation of stopping potential with fre...
Text Solution
|
- An electron of mass 'm' is moving with initial velocity v0hati in an ...
Text Solution
|
- The energy that should be added to an electron to reduce its de Brogli...
Text Solution
|
- In a photomissive cell with executing wavelength lamda//2 , work func...
Text Solution
|
- Representing the stopping potential V along X - axis and 1 / lamda alo...
Text Solution
|
- A monochromatic source of light is placed at a distance d from metal p...
Text Solution
|