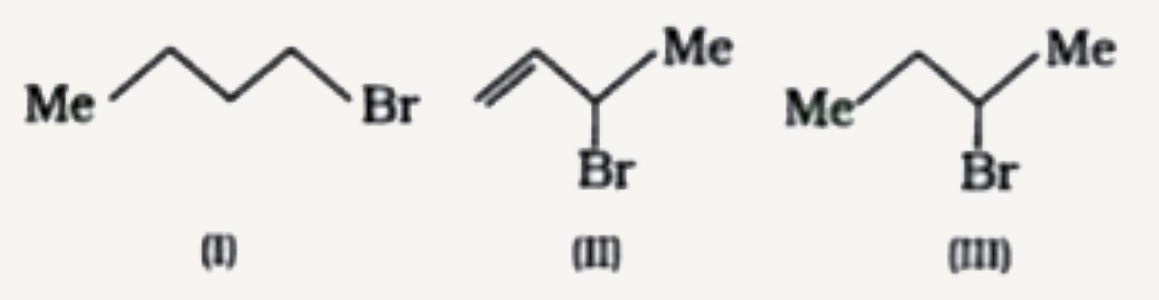
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise RECENT EXAMINATION QUESTIONS|12 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise MCQ (LEVEL-II)|98 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MODERN PUBLICATION|Exercise Recent Examination Questions|13 VideosHYDROCARBONS
MODERN PUBLICATION|Exercise RECENT EXAMINATION QUESTIONS|9 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-HALOALKANES AND HALOARENES -MCQ (LEVEL-III)
- Consider the following bromides : The correct ordder of S(N)1 rea...
Text Solution
|
- Iodoform can be prepared from all, except
Text Solution
|
- A solution of (-) -1-chloro-1-phenylethane in toluene racemises slowly...
Text Solution
|
- In S(N)2 reactions, the correct order of reactivity for the following ...
Text Solution
|
- The synthesis of alkyl fluorides is best accomplished by :
Text Solution
|