Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise MEMORY TEST (B. Complete the missing links)|11 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise MEMORY TEST (C. Choose the correct alternative)|12 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise NCERT examplar Problems (Assertion and Reason Type Questions)|10 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MODERN PUBLICATION|Exercise COMPETION FILE (Integer Type Numerical Value Type Questions)|5 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|5 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-HALOALKANES AND HALOARENES-MEMORY TEST (A. Say True or False)
- The dipole moment of CH(3)F is greater than that of CH(3)Cl.
Text Solution
|
- In general, alkyl halides are more reactive than aryl halides.
Text Solution
|
- CH(3)CH(2)I is more reactive than CH(3)CH(2)Cl towards KCN.
Text Solution
|
- Carbon tetrachloride is inflammable.
Text Solution
|
- CH(3)CH=CHCl is more/less reactive than CICH(2)CH=CH(2)?.
Text Solution
|
- Give True or False. 2,3,4- Trichloropentane has three asymmetric carb...
Text Solution
|
- Addition of BrC CI(3) to propene in the presence of peroxides gives 3-...
Text Solution
|
- Iodide is a better nucleophile than bromide.Explain
Text Solution
|
- Chlorobenzene gives a white precipitate with alcoholic silver nitrate ...
Text Solution
|
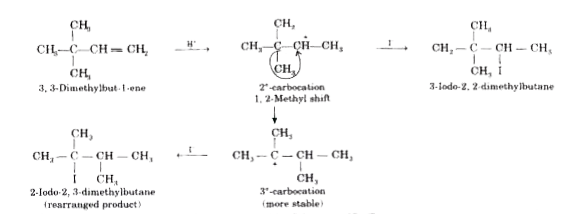
- Explain why the addition of HI to 3,3-dimethylbut-1-ene gives 2-iodo-2...
Text Solution
|
- Cheak Whatever given statement is true or False .Bromoethane reacts wi...
Text Solution
|
- 1, 1-Dichloroethane reacts with aqueous KOH to give ethanal.
Text Solution
|
- Thioethers are obtained by reacting alkyl halides with sodium hydrosul...
Text Solution
|
- Boiling point of iodobenzene is more than that of bromobenzene. true o...
Text Solution
|