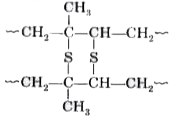
Text Solution
Verified by Experts
Topper's Solved these Questions
POLYMERS
MODERN PUBLICATION|Exercise NCERT (EXemplar Problems) (Matching Type Questions)|8 VideosPOLYMERS
MODERN PUBLICATION|Exercise NCERT (EXemplar Problems) (Assertion and Reason Type Questions)|7 VideosPOLYMERS
MODERN PUBLICATION|Exercise NCERT (EXemplar Problems) (Multiple Choice Questions (Type-II))|11 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|5 VideosSOLID STATE
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-POLYMERS-NCERT (EXemplar Problems) (Short Answer Type Questions)
- A natural linear polymer of 2 methyl -1,3 - butadiene becomes hard on ...
Text Solution
|
- Identify the type of polymer. -A-A-A-A-A-A-
Text Solution
|
- Identify the type of polymer -A-B-B-A-A-A-B-A-
Text Solution
|
- out of chain growth polymerisation and step growth polymerisation,in w...
Text Solution
|
- Identify the type of polymer given in the following figure.
Text Solution
|
- Identify the polymer given below :
Text Solution
|
- Why are rubber called elastomers?
Text Solution
|
- Can enzyme be called a polymer?
Text Solution
|
- Can nucleic acid protein and starch be considered as step growth polym...
Text Solution
|
- How is the following resin intermediate prepared and which polymers is...
Text Solution
|
- To have practical applications why are cross links quetioined in rubb...
Text Solution
|
- Why does cis polyisoprene posses elastic porperty?
Text Solution
|
- What is the structural difference between HDP and LDP? How does the st...
Text Solution
|
- What is the role of benzoyl peroxide in addition polymerisation of alk...
Text Solution
|
- Which factor imparts crystalline nature to a polymer like nylon?
Text Solution
|
- Name the polymer used in laminating sheets and give the name of monome...
Text Solution
|
- Which type of biomolecules have some structural similarity with synthe...
Text Solution
|
- Why should the monomer used in addition polymerisation through free r...
Text Solution
|