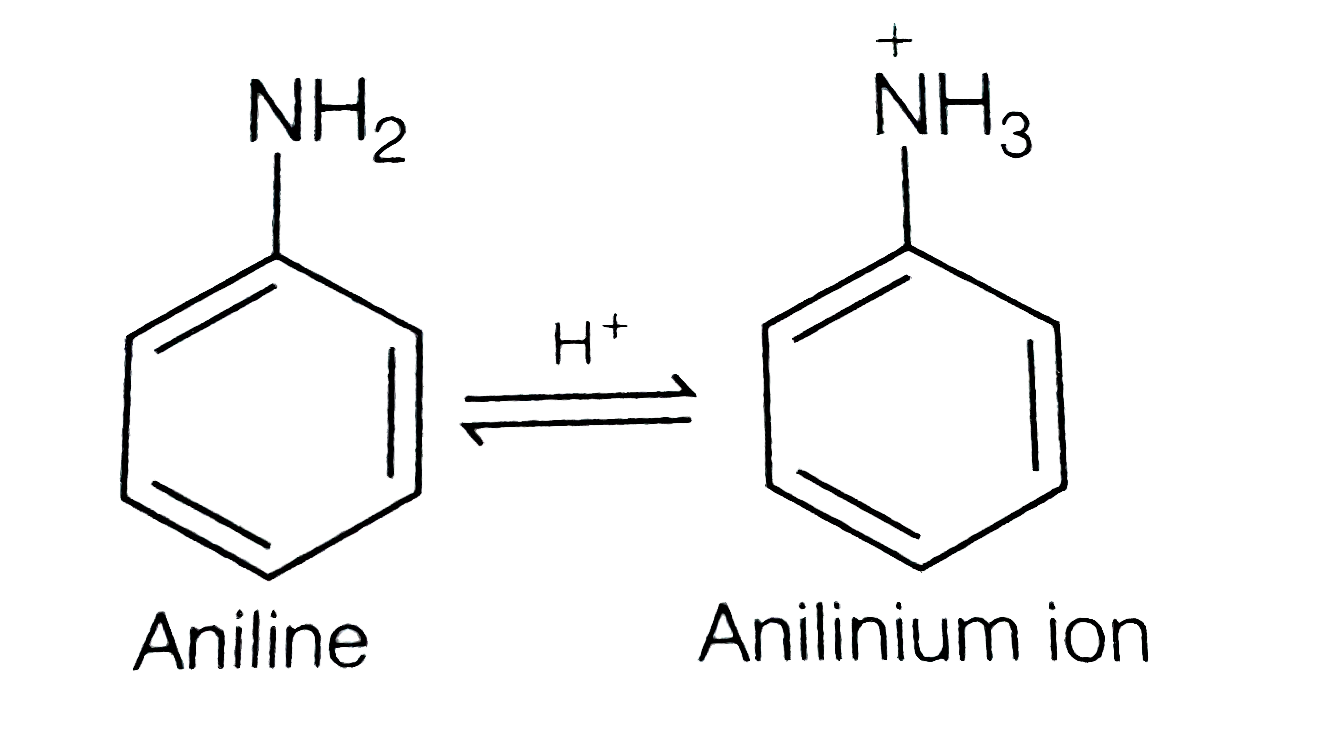
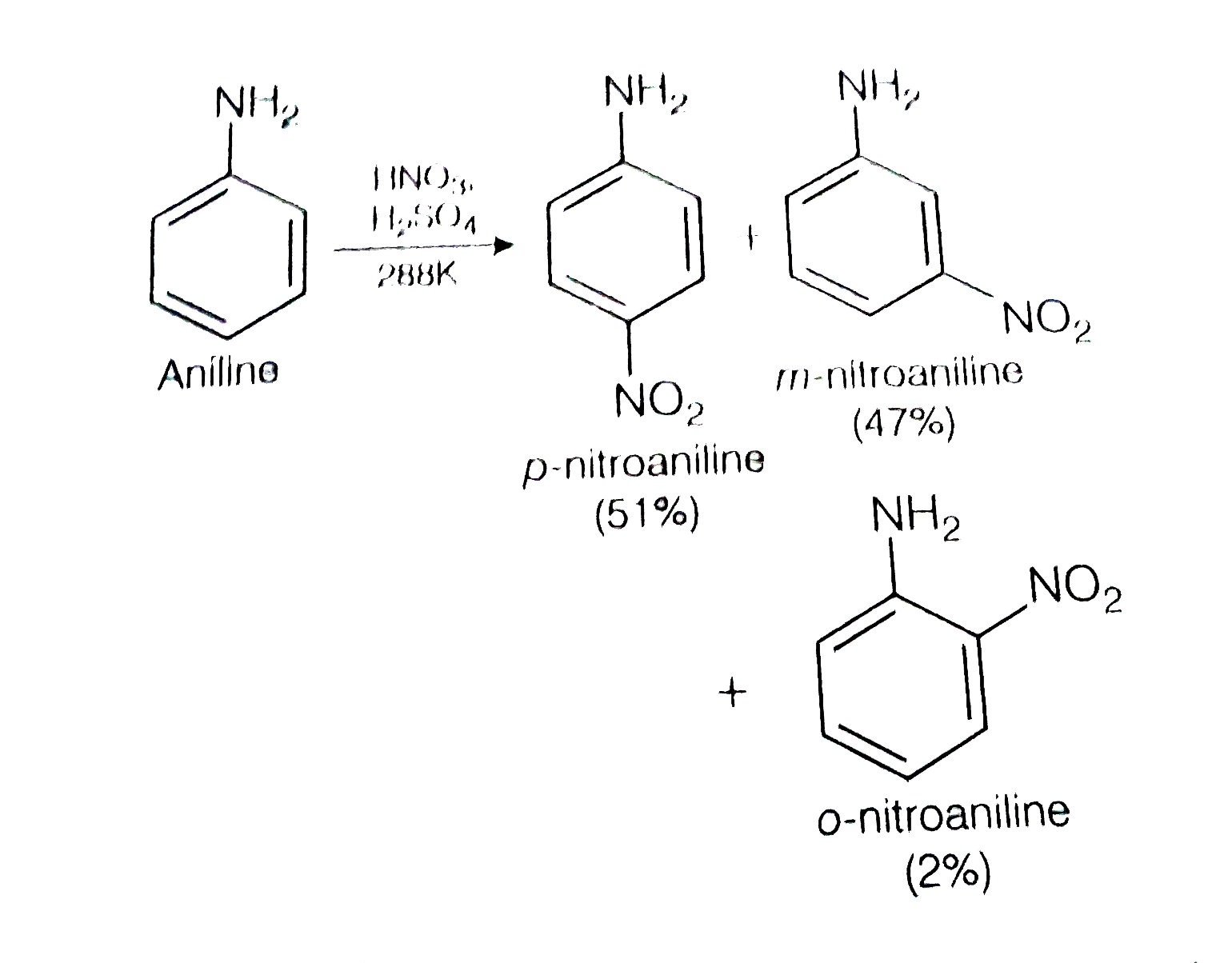
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Nitration of aniline in strong acidic medium also gives m-nitroaniline...
Text Solution
|
- Nitration of aniline also gives m-nitro anilime, in stron acidic mediu...
Text Solution
|
- Nitration of aniline in strong acidic medium also gives m-nitroaniline...
Text Solution
|
- Nitration of aniline in strongly acidic medium, results in the formati...
Text Solution
|
- Nitration of aniline in strong acidic medium also gives m-nitroaniline...
Text Solution
|
- प्रबल अम्लीय माध्यम में ऐनिलीन के नाइट्रीकरण में m-नाइट्रोऐनिलिन भी प्...
Text Solution
|
- Nitration of aniline also gives m-nitro anilime, in stron acidic mediu...
Text Solution
|
- Nitration of aniline in strong acidic medium also gives m-nitroaniline...
Text Solution
|
- Nitration of aniline in strong acidic medium also gives m-nitroaniline...
Text Solution
|