(A) In `E_(2)`-reaction `CH_(3)-CH(Br)C-D_(3)` gives
`CH_(2)=CH-CD_(3)` ltbr. `CH_(3)-underset(Br)underset(|)(CH)-CD_(3)underset("Boil")overset("Alcoh. KOH")rarr`
`CH_(2)=CH-CD_(3)+KBr+H_(2)O`
The formation of `CH_(2)=CH-CD_(3)` can be explained on the basis of the fact that. C-D bond is much stronger than C-H bond.
(B) Reactivity of `PhCHBrCH_(3)` is greater than `PhCHBrCD_(3)` because C-D bond is more stronger than C-H bond.
(C)
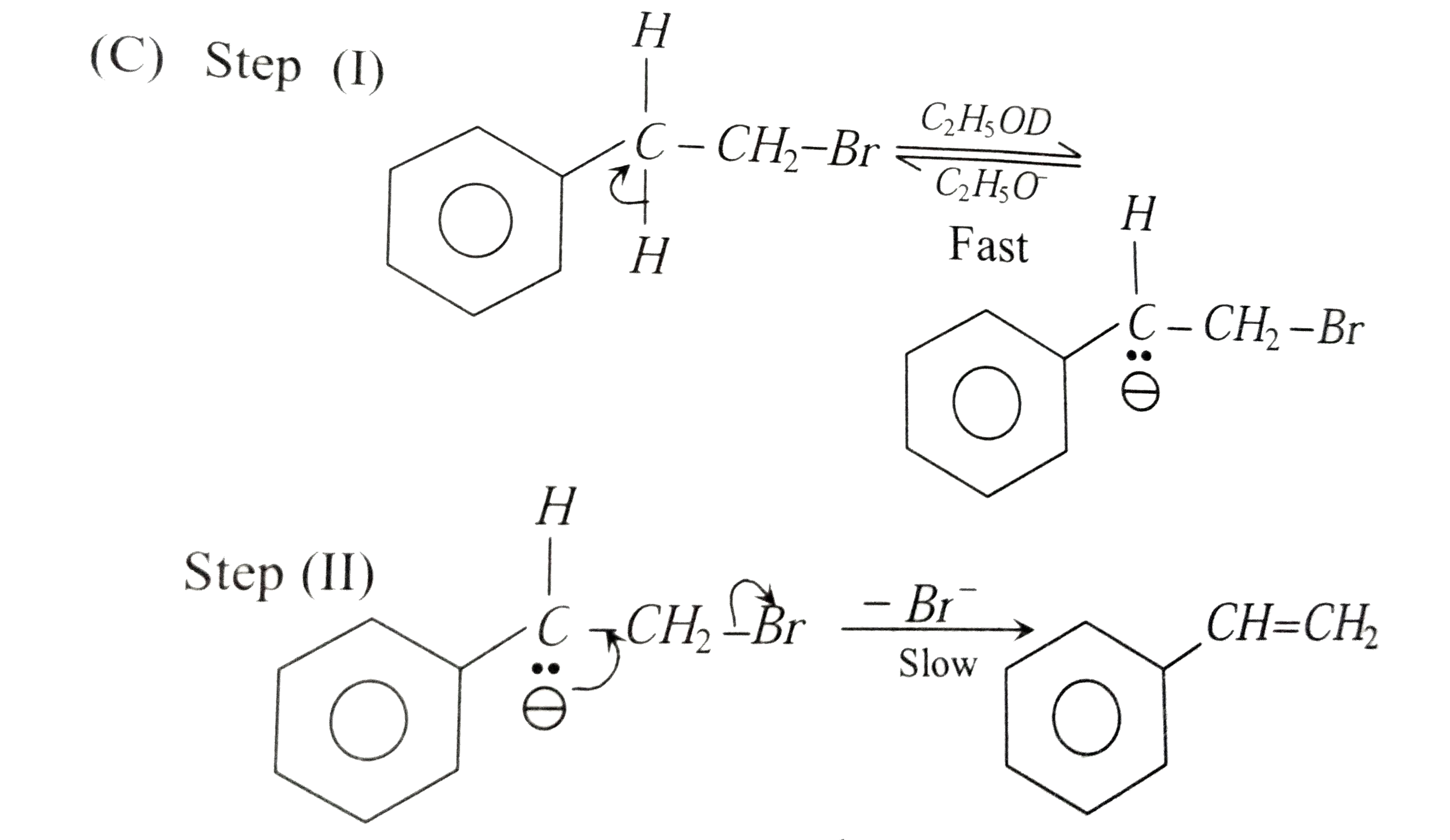
In the step (II), a slow unimolecular elimination occurs in the conjugate base of the reactant and hence this mechanism is called `E_(1cb)` or carbanion mechanism. Since step (I) must be reversible, if ethanol containing `C_(2)H_(5)OD` is used as solvent, it would be expected that the original bromide would incorporate deuterium (D).
(D) Step I. `PhCH_(2)-CH_(2)-Br overset("slow")rarr PhCH_(2)-overset(+)(C)H_(2)+Br^(-)`
Step II. `PhCH_(2) - overset(+)(C)H_(2)overset("Fast")rarr Ph-CH = CH_(2)+H^(+)`
Rate `prop [PhCH_(2)-CH_(2)-Br]` Similarly
Step I. `PhCD_(2)CH_(2)Br overset("Slow")rarr PhCD_(2)overset(+)(C)H_(2) + Br^(-)`
Step II. `PhCD_(2) overset(+)(C)H_(2) overset("Fast")rarr PhCD = CHD + H^(+)`
Rate `prop [PhCD_(2) - CH_(2)Br]`
Hence, `E_(1)` reaction and first order kinetics.

