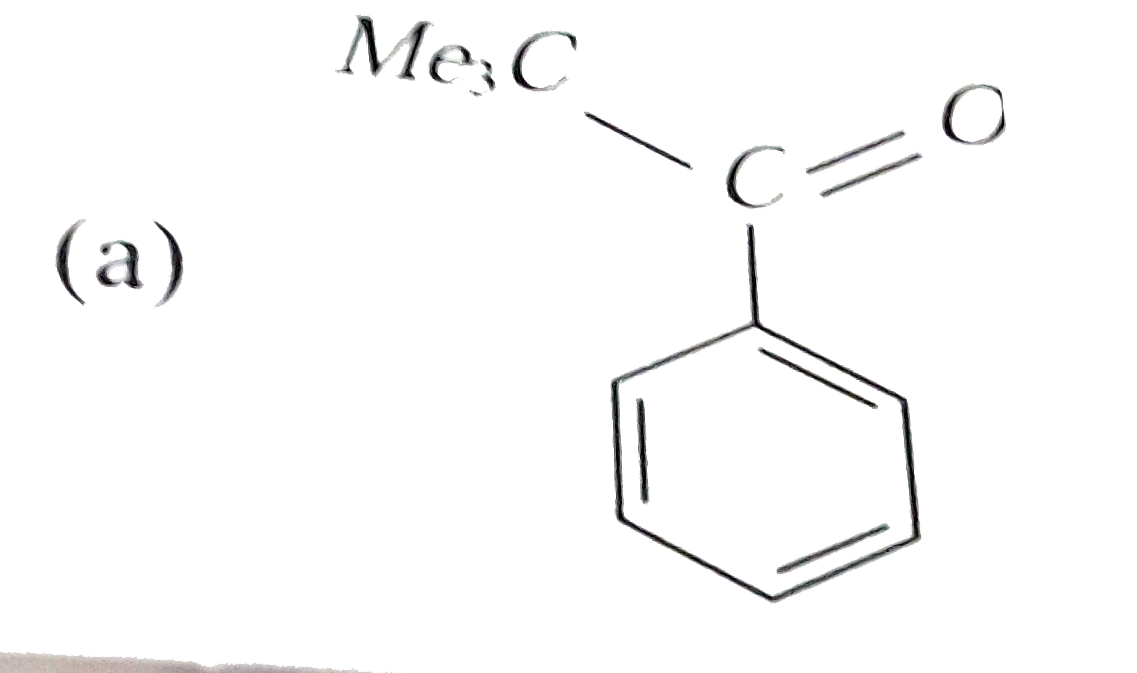
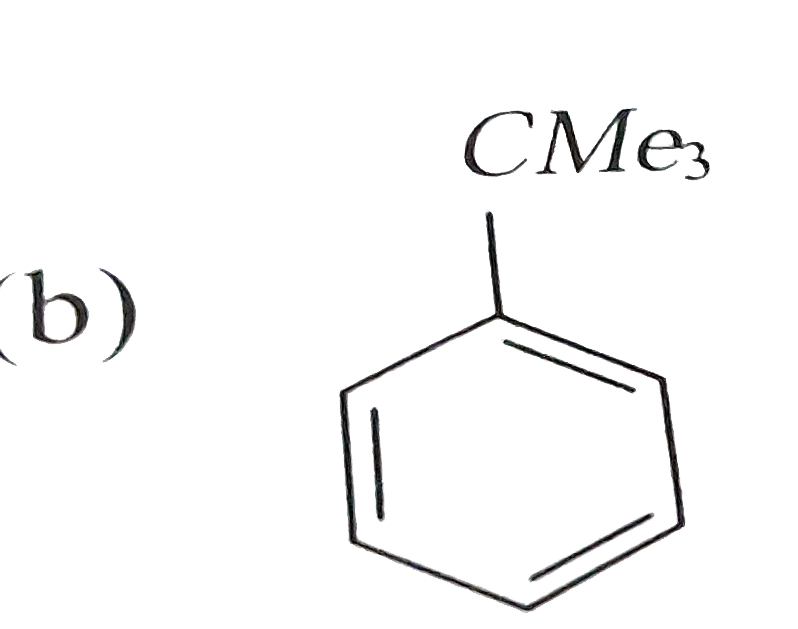
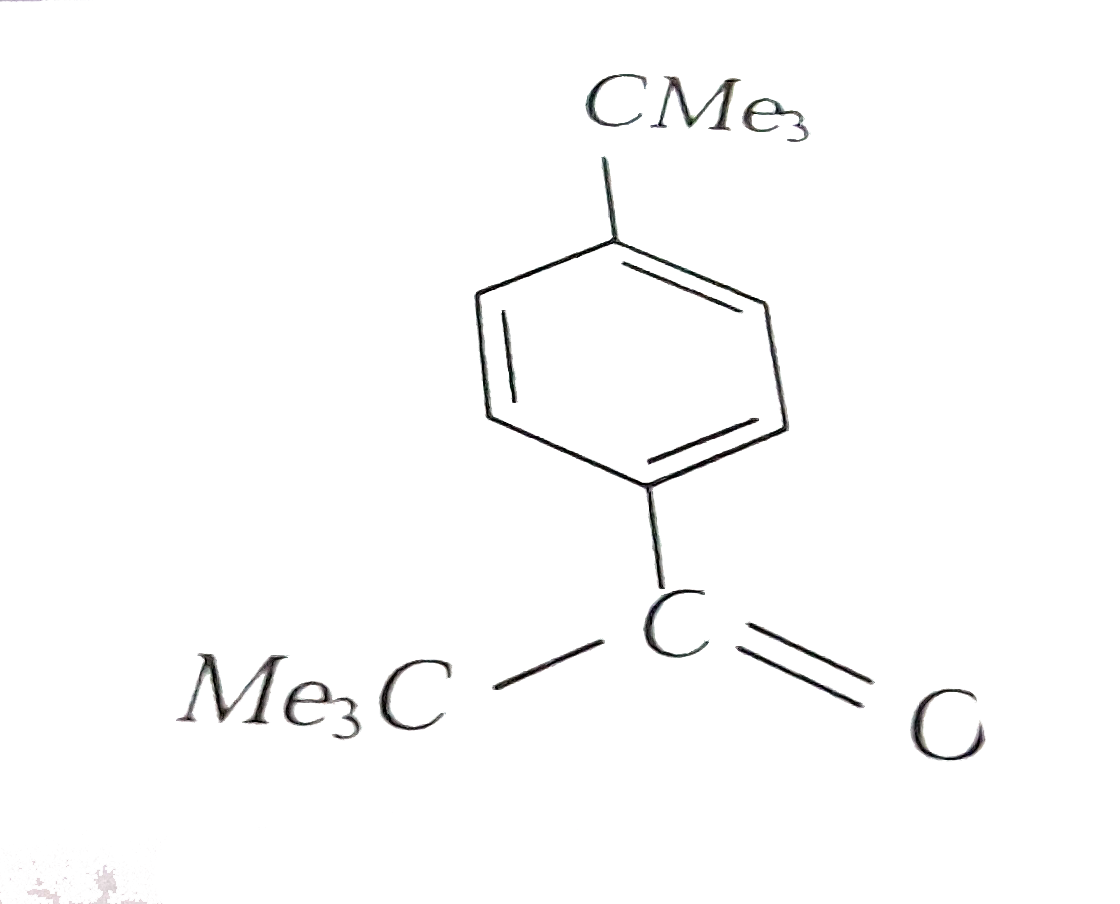
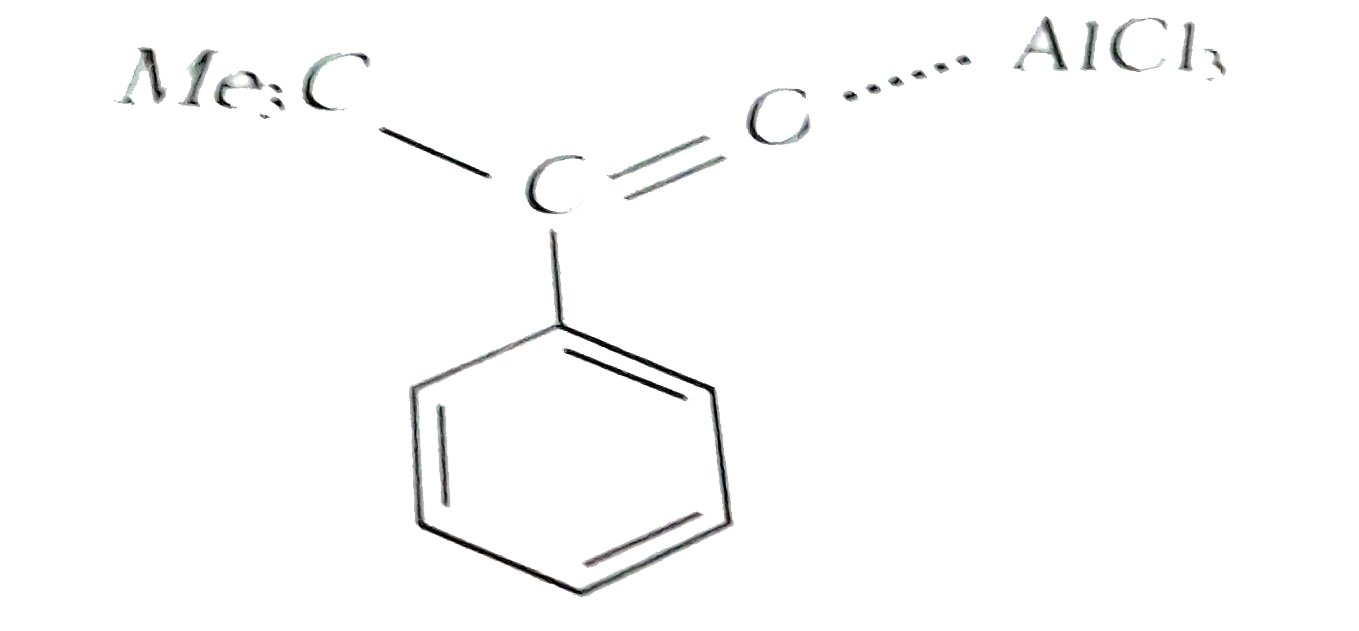
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Reaction of benzene with Me3COCl in the presence of anhydrous AlCl3 gi...
Text Solution
|
- Reaction of benzene with isobutylchloride (CH3CH(CH3)CH2Cl) in the pre...
Text Solution
|
- Reaction of benzene with Me3COCl in the presence of anhydrous AlCl3 gi...
Text Solution
|
- The reactions of benzene with acetic anhydride in presence of anhydrou...
Text Solution
|
- Reactions of benzene with Me3C COCl in the presence of anhydrous AlCl3...
Text Solution
|
- Ansisole on reaction ith chloromethane in presence of anhydrous AlCl3 ...
Text Solution
|
- Reaction of benzene with Me3C""COCl in the presence of anhydrous AlCl3...
Text Solution
|
- Reaction of benzene with alkyl halide in the presence of anhydrous AlC...
Text Solution
|
- Ansisole on reaction ith chloromethane in presence of anhydrous AlCl3 ...
Text Solution
|