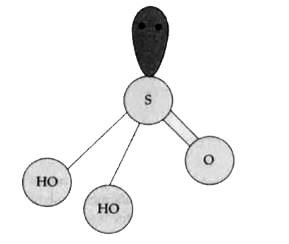
Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC ANNUAL EXAMINATION 2019
OSWAAL PUBLICATION|Exercise PART - D|10 VideosII PUC ANNUAL EXAMINATION 2019
OSWAAL PUBLICATION|Exercise PART - B|8 VideosHALOALKANES & HALOARENES
OSWAAL PUBLICATION|Exercise Topic 2 (Properties of Haloarenes and Haloalkanes) (Long Answer Type Questions - II)|1 VideosII PUC APRIL - 2016
OSWAAL PUBLICATION|Exercise PART - D|27 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-II PUC ANNUAL EXAMINATION 2019 -PART - C
- (a) In extraction of Aluminium by electrolysis, (i) Write ovreall ce...
Text Solution
|
- In the manufacture of ammonia by Haber's process. Write the flow chart...
Text Solution
|
- Answer any five of the following questions. a) Given reason: (i) Hyd...
Text Solution
|
- Complete the following chemical equations : (i) NH3 + 3CI2 to … + 3H...
Text Solution
|
- Write the balanced chemical equation involved in the manufacture of po...
Text Solution
|
- Transition elements shows catalytic property. Give two reason.
Text Solution
|
- Give the IUPAC name of K3 [Cr(C2 O4)3]
Text Solution
|
- Using VBT, explainthe geometry and magnetic property of [Ni(CN)(4)]^(-...
Text Solution
|