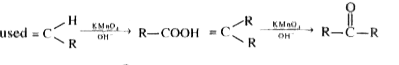A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-1 (More than one correct answer questions)|5 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-1 (Linked comprehension Type Questions)|6 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise LEVEL-II LEXTURE SHEET (Exericse-IV) (Integer answer type Questions)|9 VideosCARBOHYDRATES
AAKASH SERIES|Exercise Practice Sheet-5|30 VideosCARBOXYLIC ACIDS AND DERIVATIVES
AAKASH SERIES|Exercise CONVERSIONS|19 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CARBOXYLIC ACIDS -PRACTICE SHEET-1 (Single answer questions)
- Oxidation of (CH3)2 C-CHCH2 CH3 with hot alkaline KMnO4 gives
Text Solution
|
- The calcium salt of which of the following acids on dry distillation p...
Text Solution
|
- Acetaldehyde overset(HCN)to X underset(H2 O //H^+)to Y overset("Heat"...
Text Solution
|
- The compound obtained by reaction of CO with NaOH followed by nutralis...
Text Solution
|
- Which of the following has highest solubility in water
Text Solution
|
- CH3 OH overset(PCl5)to (A) overset(KCN)to (B) overset(H3 O^+)to (C) th...
Text Solution
|
- The end product (C) in the following sequence of reactions, CH3 Cl...
Text Solution
|
- Which of the following is optically active
Text Solution
|
- In the series of reaction CH3 COOH overset(NH3)to A overset(Delta)to B...
Text Solution
|
- Which of the following anions is not stabilised by resonance
Text Solution
|
- The major product which can be isolated from this reaction is
Text Solution
|