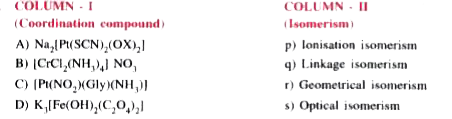Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 3 (Single or more than one option questions)|16 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 3 ( Linked Comprehension type questions Passage - 1:)|4 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 2 ( Linked Comprehension type questions Passage -II :)|3 VideosCHEMISTRY IN EVERYDAY LIFE
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (PRACTICE SHEET -2 (INTEGER ANSWER TYPE QUESTIONS))|7 VideosCOMPLEX COMPOUNDS
AAKASH SERIES|Exercise PRACTICE EXERCISE|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CO-ORDINATION COMPOUNDS-PRACTICE SHEET - 2 (Match the following questions )
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- The number of isomers possible for square planar complex K(2)[PdClBr(2...
Text Solution
|
- Number of isomers possible for [Co(en)(2)Cl(NO(2))]Cl are
Text Solution
|
- Find out maximum number of geometrical isomers theoritically possible ...
Text Solution
|
- No. of isomers of [Co(NH(3))(2)Cl(2)(en)]^(+) are
Text Solution
|
- No. of geometrical isomers of [RhCl(CO)(PPh(3))(NH(3)) are
Text Solution
|
- The possible no. of coordination isomers of [Cr(NH(3))(6)][Co(CN)(6)] ...
Text Solution
|