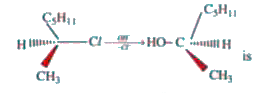A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 1 (POLYHALOGEN COMPOUNDS)|7 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 2 A (INTRODUCTION, NATURE OF C-X BOND)|20 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 1 (HALOARENES (CHLOROBENZENE))|7 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise Exercise 3.2|41 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 6 (Integer answer type Questions)|9 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALKYL AND ARYL HALIDES-OBJECTIVE EXERCISE - 1 (PROPERTIES OF CHLOROBENZENE)
- The reaction given
Text Solution
|
- C2H5Cl underset(Ag2O)overset(aq)(to) A underset(360^@C)overset(Al2O5)(...
Text Solution
|
- When an alkyl halide is heated with dry Ag2O,it produces
Text Solution
|
- On sulphonation of C6H5CI
Text Solution
|
- C6H5NH2 underset(0-5^@C)overset(NaNO2 + HCl)(rarr)A overset(KI)(rarr)B...
Text Solution
|
- Chlorobenzene on fusing with solid NaOH follwed by acidification gives
Text Solution
|
- Chlorobenzene on reaction with CH3Cl in the presence of AlCl3 will giv...
Text Solution
|
- Chlorobenzene reacts with Mg in dry ether to give a compound (A) which...
Text Solution
|
- The reaction given below is known as C6H5I + 2Na + ICH3 rarrC6H5 -CH3+...
Text Solution
|
- Halide most readily hydrolyses is (SN1)
Text Solution
|
- Correct statement about the electrophilic substitution in benzene ring...
Text Solution
|
- Which one of the following is most reactive in nucleophilic substituti...
Text Solution
|