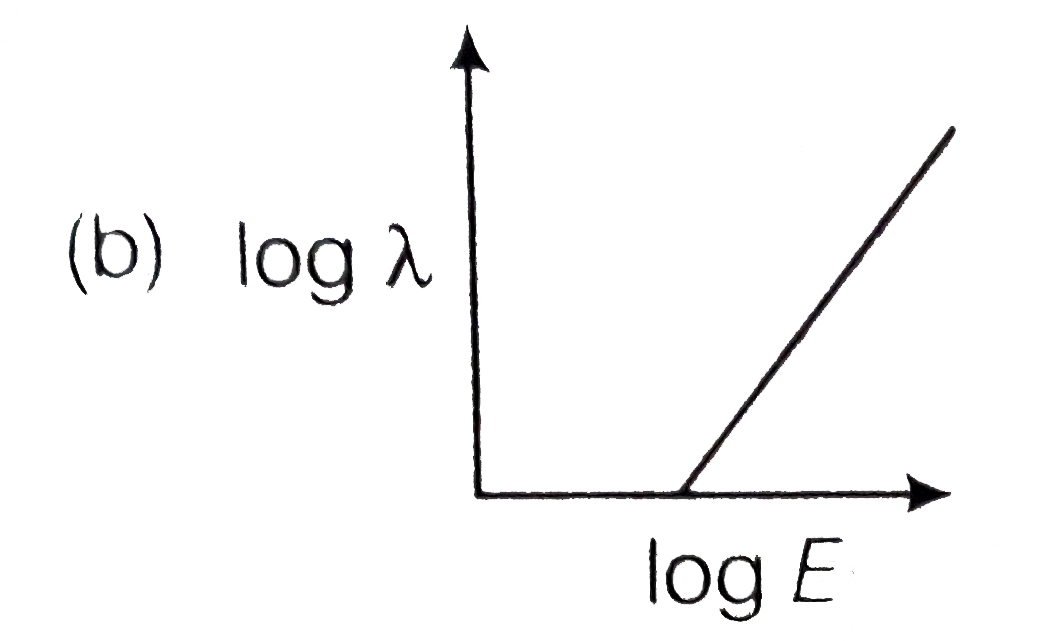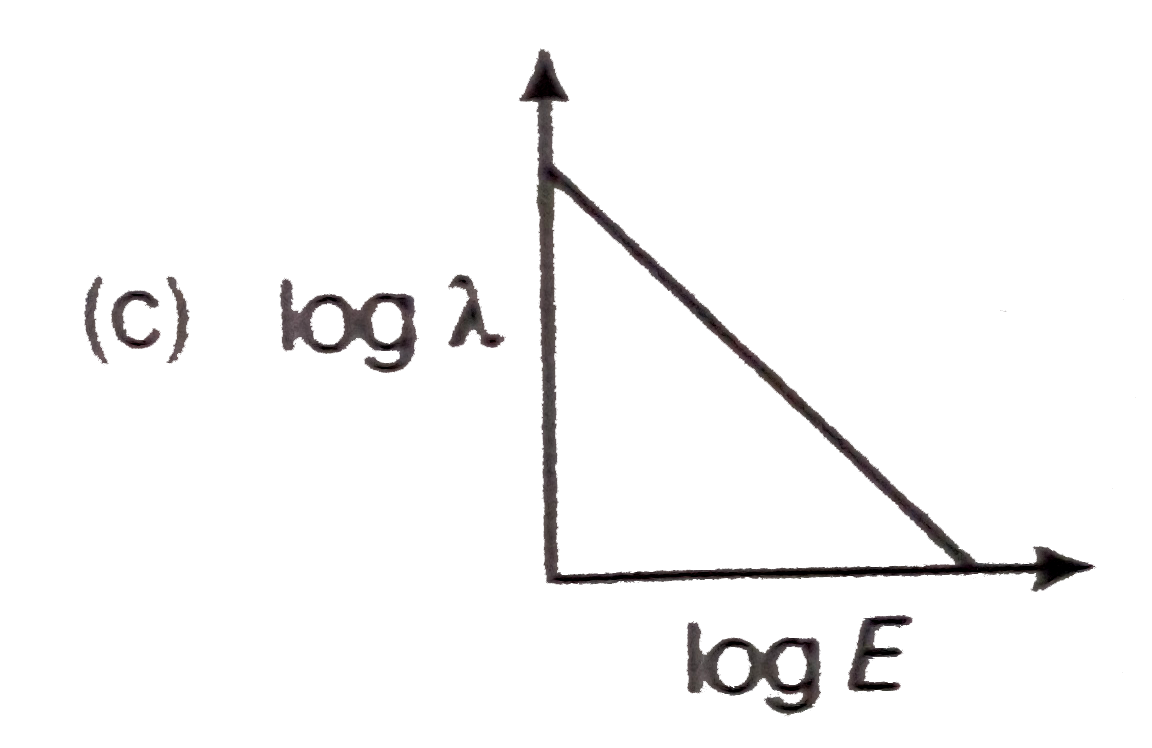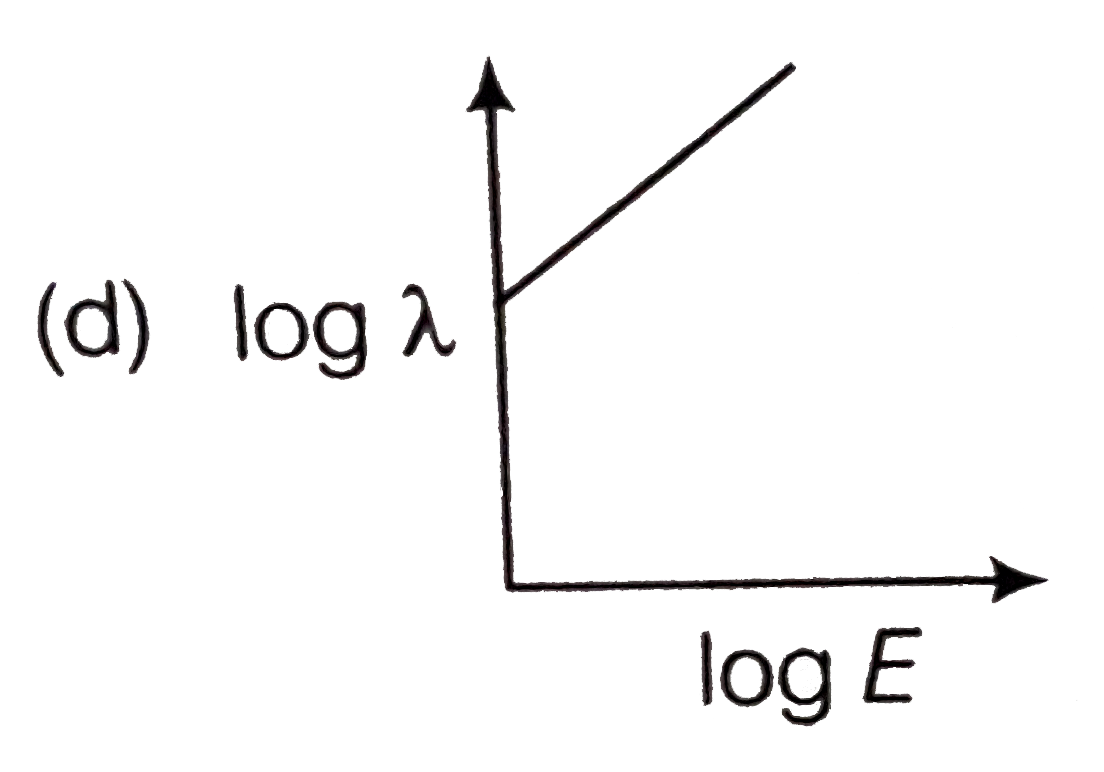A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SOLVED PAPERS 2018
DC PANDEY ENGLISH|Exercise Assertion and Reasons|10 VideosSOLVED PAPERS 2018
DC PANDEY ENGLISH|Exercise JIPMER|22 VideosSOLVED PAPERS 2018
DC PANDEY ENGLISH|Exercise JIPMER|22 VideosSOLVED PAPER 2017
DC PANDEY ENGLISH|Exercise Solved papers 2017(JIPMER)|32 VideosWAVE OPTICS
DC PANDEY ENGLISH|Exercise For JEE Advanced E. Integer Type Questions|13 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY ENGLISH-SOLVED PAPERS 2018-AIIMS
- Positive charge Q is distributed uniformly over a circular ring of rad...
Text Solution
|
- An infinite number of identical capacitors each of capacitance 1 mF ar...
Text Solution
|
- In the circuit in fig. If no current flows through the galvanometer wh...
Text Solution
|
- In a series C-R circuit shown in figureure, the applied voltage is 10 ...
Text Solution
|
- A system S consists of two coils A and B. The coil, A carries a steady...
Text Solution
|
- A long straight wire, carrying current I, is bent at its midpoint to f...
Text Solution
|
- An element dvecl=dxhati (where dx=1cm) is placed at the origin and car...
Text Solution
|
- The coil in figure carries current i=2.00 A in the direction indicated...
Text Solution
|
- Consider the following figure, a uniform magnetic field of 0.2 T is di...
Text Solution
|
- An idal coil of 10 is connected in series with a resitance of 5Omega a...
Text Solution
|
- In the circuit, shown the galvanometer G of resistance 60Omega is shif...
Text Solution
|
- In a circuit L, C and R are connected in series with an alternating vo...
Text Solution
|
- The log - log graph between the energy E of an electron and its de - B...
Text Solution
|
- The half life of a radioactive substance is 20 minutes . The approxima...
Text Solution
|
- The diode used at a constant potential drop of 0.5 V at all currents a...
Text Solution
|
- An upolarised beam of intensity 2a^(2) passes through a thin polarioid...
Text Solution
|
- A hydrogen like atom of atomic number Z is in and excited state of qua...
Text Solution
|
- A diode detector is used to detect an amplitude modulated wave of 60% ...
Text Solution
|
- A circular loop of radius 0.3 cm lies parallel to amuch bigger circula...
Text Solution
|
- In the adjoining circuit diagram, the readings of ammeter and voltmete...
Text Solution
|