Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2016)
SUNSTAR PUBLICATION|Exercise PART - C|12 VideosII PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2014)
SUNSTAR PUBLICATION|Exercise PART - D|22 VideosII PUC CHEMISTRY SUPPLEMENTARY EXAM QUESTION PAPER JUNE -2019
SUNSTAR PUBLICATION|Exercise PART-D|34 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2016)-PART - D
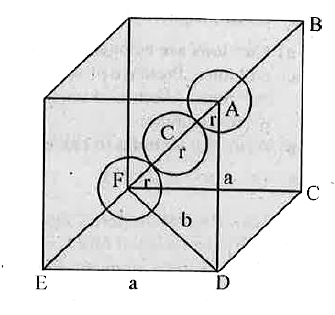
- a) Calculate the packing efficiency of particles in a body centred cub...
Text Solution
|
- Calculate the number of paricles per unit cell of FCC .
Text Solution
|
- 300cm^(3) of an aqueous solution of a protein contains 2.12 g of the p...
Text Solution
|
- i) State Henry's law. ii) Soda water bottles are sealed under high p...
Text Solution
|
- Find the value of AG^(@) at 25^(@)C for the following electrochemical ...
Text Solution
|
- Write the equations of anodic and cathodic reactions occur during rust...
Text Solution
|
- Derive an integrated rate equation for the rate constant of a zero ord...
Text Solution
|
- Write the energy distribution curve showing temperature dependence of ...
Text Solution
|
- Mention any one application of adsorption.
Text Solution
|
- i) What is 'Tyndall effect'? ii) In the coagulation of negative sol,...
Text Solution
|
- What is heterogeneous catalysis? Give an example.
Text Solution
|
- i) Explain S(N)2 mechanism taking an example of chloromethane. ii) W...
Text Solution
|
- i) CH(3)-Br+AgFrarr CH(4)F+AgBr Name the above reaction. ii) P - d...
Text Solution
|
- i) Identifiy 'A' and 'B' in the following equations. CH(3)-CH=CH(2) ...
Text Solution
|
- Explain Williamson's ether synthesis.
Text Solution
|
- i) How does benzaldehyde reacts with acetophenone in presence of a dil...
Text Solution
|
- Among formic acid and acetic acid, which is more acidic ? Give reason...
Text Solution
|
- i) Explain the reduction of nitrocompounds to amines with an examples....
Text Solution
|
- How is aniline converted in phenyl isocyanide ? Write the equation.
Text Solution
|
- Write Haworth structure for maltose.
Text Solution
|