Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2016)
SUNSTAR PUBLICATION|Exercise PART - D|25 VideosII PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2016)
SUNSTAR PUBLICATION|Exercise PART - B|8 VideosII PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2014)
SUNSTAR PUBLICATION|Exercise PART - D|22 VideosII PUC CHEMISTRY SUPPLEMENTARY EXAM QUESTION PAPER JUNE -2019
SUNSTAR PUBLICATION|Exercise PART-D|34 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2016)-PART - C
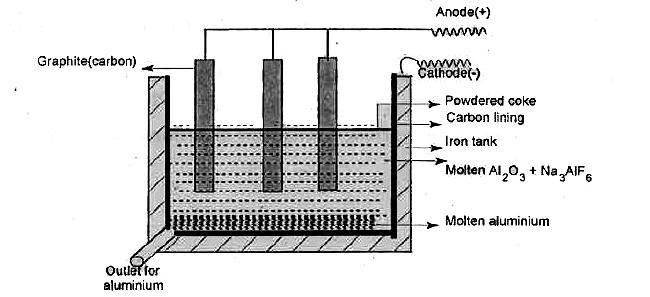
- Explain the extraction of aluminium from purified alumina by Hall-Hero...
Text Solution
|
- Write the balanced chemical equation with condition involved in the ma...
Text Solution
|
- Mention three anomalouos behaviour of oxygen.
Text Solution
|
- Write the structure of Sulphuric acid.
Text Solution
|
- How hot and concentrated sodium hydroxide reacts with chlorine gas ?
Text Solution
|
- How does electronegativity of Halogens vary down the group?
Text Solution
|
- Cu^(2+) ions are coloured but Zn^(2+) ions are colourless. Give reason...
Text Solution
|
- Write the formula to calculate spin only magnetic moment.
Text Solution
|
- How is KMnO(4) [Potassium permanganate] is prepared from MnO(2)? Write...
Text Solution
|
- Using VBT, explain the geometry and magnetic property of [CO(NH(3))(6)...
Text Solution
|
- (a) Explain ionozation isomerism with an example .
Text Solution
|
- What are homoleptic complexes?
Text Solution
|