Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)
SUNSTAR PUBLICATION|Exercise PART - C|11 VideosII PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)
SUNSTAR PUBLICATION|Exercise PART - D|26 VideosII PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)
SUNSTAR PUBLICATION|Exercise PART - D|26 VideosII PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2015)
SUNSTAR PUBLICATION|Exercise PART - D|26 VideosII PUC CHEMISTRY (P.U. BOARD LATEST MODEL QUESTION PAPER - 1)
SUNSTAR PUBLICATION|Exercise PART - D|25 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)-PART - B
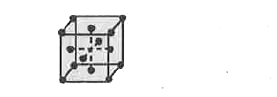
- Calculate the no. of particles (atoms) per unit cell in a FCC crystal ...
Text Solution
|
- What are ferromagnetic substances? Give one example.
Text Solution
|
- The rate constant of a certain first order reaction is 200S^(-1). What...
Text Solution
|
- Zr and Hf have almost identical atomic radii. Give reason?
Text Solution
|
- Explain Kolbe's reaction.
Text Solution
|
- What is the action of dil NaOH on ethanal (acetaldehyde) ? Name the re...
Text Solution
|
- What is the role of the following chemicals in food? (a) Sodium benzoa...
Text Solution
|
- What are antifertility drugs ? Give an example
Text Solution
|