Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
ICSE|Exercise NCERT TEXT-BOOK EXERCISES (With Hints and Solutions)|23 VideosSTATES OF MATTER : GASES AND LIQUIDS
ICSE|Exercise ASSERTION-REASON TYPE QUESTIONS|5 VideosSOME P-BLOCK ELEMENTS
ICSE|Exercise NCERT TEXTBOOK EXERCISES (With Hints and Solutions)|63 VideosSTRUCTURE OF ATOM
ICSE|Exercise NCERT Textbook Exercises|67 Videos
Similar Questions
Explore conceptually related problems
ICSE-STATES OF MATTER : GASES AND LIQUIDS-NUMERICAL PROBLEMS
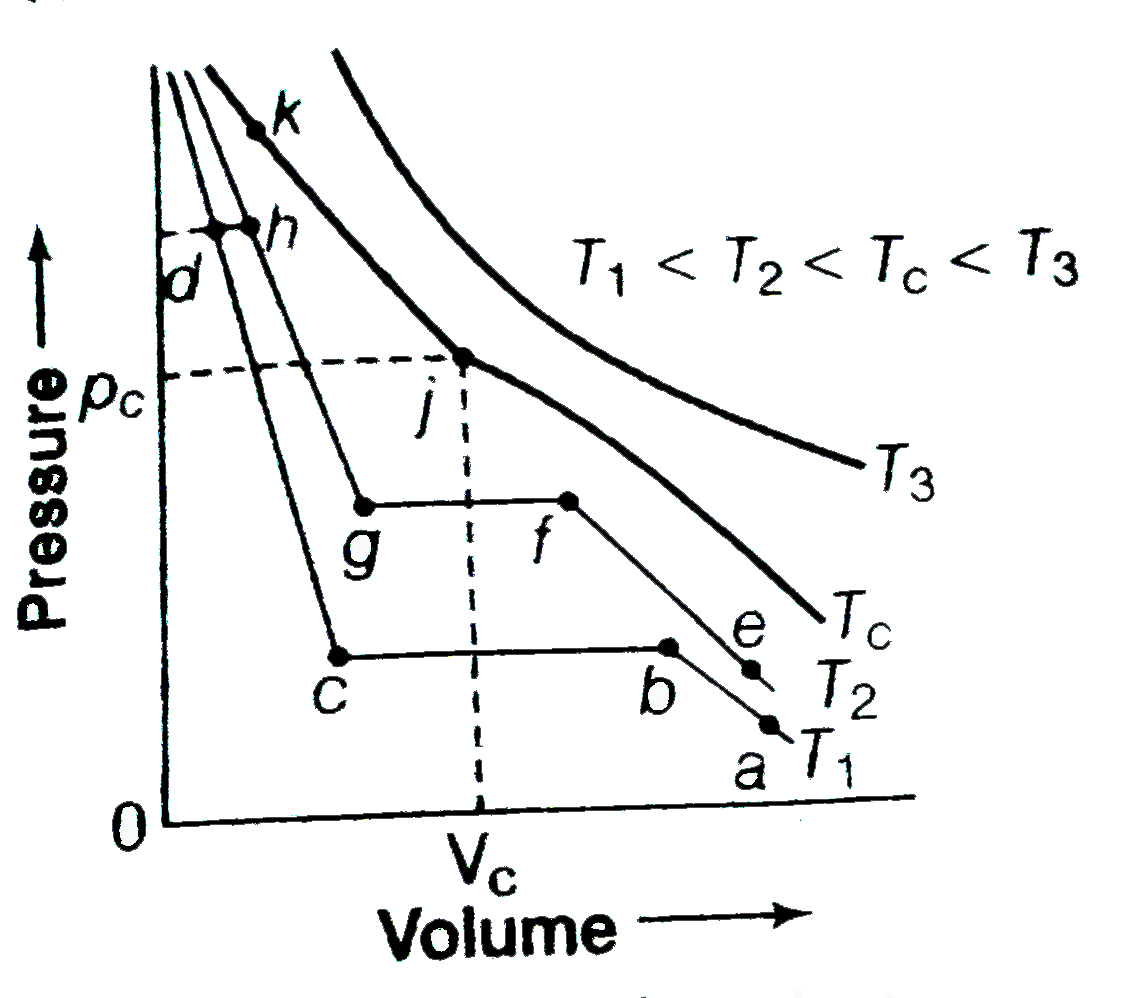
- Isotherms of carbon dioxide at various temperatures are repersented in...
Text Solution
|
- The volume of an air bubblebecomes three times as it rises from the bo...
Text Solution
|
- At S.T.P. a mixture of 280 mL of CH4 " and 140 mL of " H2 is complete...
Text Solution
|
- Calculate the pressure exerted by 0.250 moles of carbon dioxide in 0.2...
Text Solution
|
- Calculate the relative rates of diffusion of ""^235UF6 and ""^238UF6 ...
Text Solution
|
- An iron cylinder contains helium at a pressure of 250 k Pa at 300 K. T...
Text Solution
|
- A 4: 1 molar mixture of He and CH4 is contained in a vessel at 20 bar ...
Text Solution
|
- What will be the density of carbon dioxide at 100^@C and 800 mm Hg pre...
Text Solution
|
- A gas occupies 300.0 mL at 27^@C and 730 mm pressure. What would be it...
Text Solution
|
- A certain quantity of a gas occupies 300 mL when collected over water ...
Text Solution
|
- A gas bulb of 1 litre capacity contains 2.0 xx 10^(21) molecules of ni...
Text Solution
|
- In a Victor Meyer's determination, 0.23 g of a volatile substance disp...
Text Solution
|
- At room temperature, ammonia gas at 1 atm pressure and hydrogen chlori...
Text Solution
|
- An open vessel contains air at 27^@C. To what temperature should the v...
Text Solution
|
- Calculate the average kinetic energy in joules of the molecules in 8.0...
Text Solution
|
- 3 moles of a gas are present in a vessel at a temperature of 27^@C. Wh...
Text Solution
|
- 1470 cm^3 of a gas is collected over water at 303 K and 74.4 cm of Hg....
Text Solution
|
- A certain gas occupies 0.418 litres at 27^@C and 740 mm Hg. What is...
Text Solution
|
- A certain gas occupies 0.418 litres at 27^@C and 740 mm Hg. If the s...
Text Solution
|
- A certain gas occupies 0.418 litres at 27^@C and 740 mm of Hg. If we...
Text Solution
|