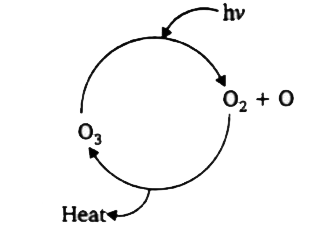
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ENVIRONMENTAL CHEMISTRY
VK JAISWAL ENGLISH|Exercise ONE OR MORE ANSWER IS/ARE CORRECT|8 VideosENVIRONMENTAL CHEMISTRY
VK JAISWAL ENGLISH|Exercise MATCH THE COLUMN|7 VideosENVIRONMENTAL CHEMISTRY
VK JAISWAL ENGLISH|Exercise Level 2|42 Videosd-BLOCK ELEMENTS
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|32 VideosMETALLURGY
VK JAISWAL ENGLISH|Exercise ASSERTION-REASON TYPE QUESTIONS|29 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL ENGLISH-ENVIRONMENTAL CHEMISTRY-Level 3
- When heathly earth's stratosphere contains a low concentration of ozon...
Text Solution
|
- When heathly earth's stratosphere contains a low concentration of ozon...
Text Solution
|
- When heathly earth's stratosphere contains a low concentration of ozon...
Text Solution
|
- When heathly earth's stratosphere contains a low concentration of ozon...
Text Solution
|