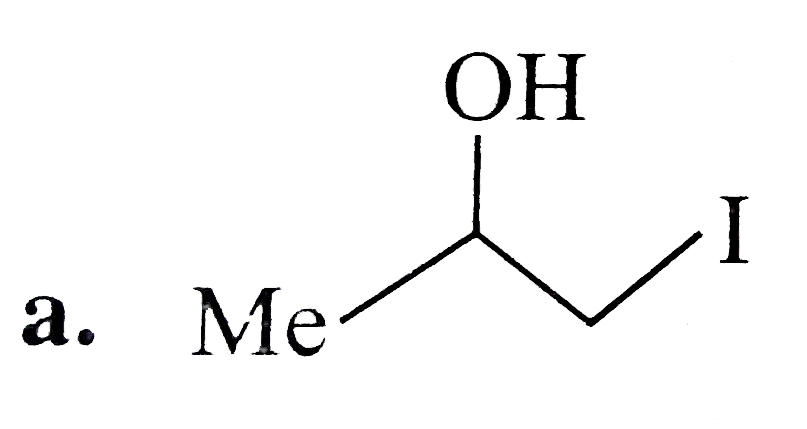
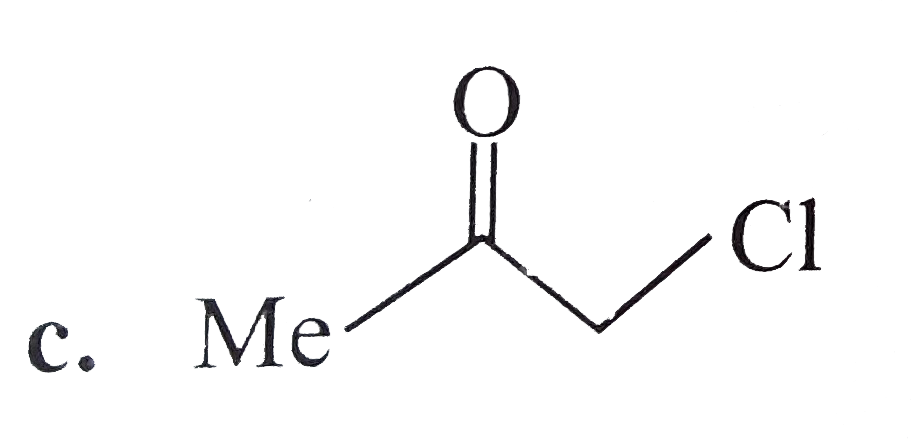
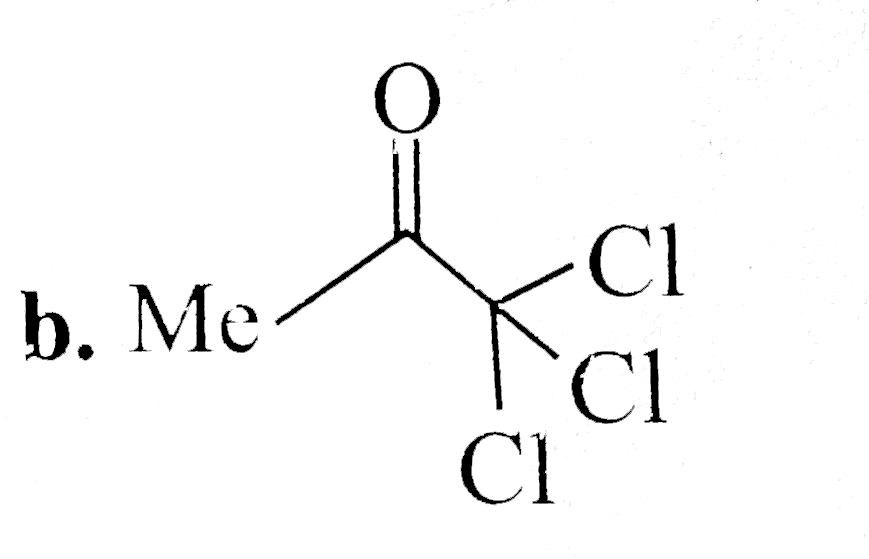A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Assertion And Reasoning)|10 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise Archives (Subjective)|26 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Multiple Correct)|35 VideosQUALITATIVE INORGANIC SALT ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Viva Voce Questions And Part-C (Analysis Of Cations)|42 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.2 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-REDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS-Exercise (Single Correct)
- Which of the following gives yellow precipitate with NaOI?
Text Solution
|
- The products (A) and (B), respectively, are:
Text Solution
|
- Compound (A) (C(4)H(8)O(3)) reacts with NaHCO(3) and evolves CO(2)(g)....
Text Solution
|
- The compound (C ) is:
Text Solution
|
- The compound (B) and (C ), respectively, are:
Text Solution
|
- The compound (A) is:
Text Solution
|
- The compound (A) is:
Text Solution
|
- The compound (A) is:
Text Solution
|
- Acid-catalysed hydration oxymercuration-demercu-ration, and hydroborat...
Text Solution
|
- The increasing order of the rate of oxidation with HIO(4) oxidation of...
Text Solution
|
- Compound (A) underset(HIO(4))overset(1 mol of)(rarr) The compound...
Text Solution
|
- Conpound (A) underset(HIO(4))overset(2 mol of)(rarr) 2 mol of glyoxali...
Text Solution
|
- The compound (B) is:
Text Solution
|
- Which type of reaction in the reduction of carbonyl compound with LAH ...
Text Solution
|
- Oxidation of aldehyde and ketone by peroxybenzoic acid to ester is cal...
Text Solution
|
- Barium adipate overset(Dry distillation)(rarr)(A)overset(MeCO(3)H)(rar...
Text Solution
|
- The products (A) is:
Text Solution
|
- The products (A), (B), and (C ) are:
Text Solution
|
- The products (A), (B), and (C ) are:
Text Solution
|
- For the following reaction, which of the following sttaments is correc...
Text Solution
|