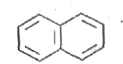
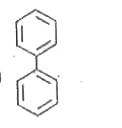
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section - B) (Obejctive type question)|5 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment(Section - C) (Previous Years Questions)|60 VideosHYDROCARBONS
AAKASH INSTITUTE ENGLISH|Exercise Exercise|33 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT SECTION -D|15 VideosHYDROGEN
AAKASH INSTITUTE ENGLISH|Exercise ASSIGNMENT (SECTION - D) (Assertion-Reason Type question)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-HYDROCARBONS-Assignment(Section - A) (Obejctive type question)
- Which one is most stable ?
Text Solution
|
- Which one is not aromatic compound ?
Text Solution
|
- The incorrect match is
Text Solution
|
- Which of the following statement is not correct for sigma and pi-bonds...
Text Solution
|
- The possible compound A is
Text Solution
|
- Which one of the following cannot be prepared by Wurtz reaction?
Text Solution
|
- The reaction of CH(3)CH=CH(2) with HOCl will yield
Text Solution
|
- C(6)H(5)CH(2)CH(2)CH(3) is when oxidised in the presence of alk. KMnO(...
Text Solution
|
- Toulene overset(K(2)Cr(2)O(7))underset(H(2)SO(4))to Y. Here Y is
Text Solution
|
- C(6)H(6)+Z overset(Anhy.AlCl(3))to Toluene The compound Z is
Text Solution
|
- C(6)H(6) overset("Oxidation")underset(V(2)O(5)//Delta)to X. Here, X is
Text Solution
|
- In kharash effect,reaction follows
Text Solution
|
- A,B and C can be
Text Solution
|
- Benzene undergoes substituion reaction more easily than addition becau...
Text Solution
|
- A mixture of C(2)H(6), C(2)H(4) and C(2)H(2) is bubbled through alkali...
Text Solution
|
- Ethylene reacts with S(2)Cl(2) to give
Text Solution
|
- Ph-C-=CH overset(Hg^(+),H^(+)//H(2)O)to A Aditon of H(2)O in the rea...
Text Solution
|
- Monomer of neoprene is
Text Solution
|
- Compound A is :
Text Solution
|
- Compound A is :
Text Solution
|