Text Solution
Verified by Experts
Topper's Solved these Questions
P BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise Exercise 1 part 1 subjective ques|30 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise Exercise 1 part 2 objective que|48 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise Example|30 VideosNUCLEAR CHEMISTRY
RESONANCE ENGLISH|Exercise STAGE-II|1 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-P BLOCK ELEMENTS-B.L.E
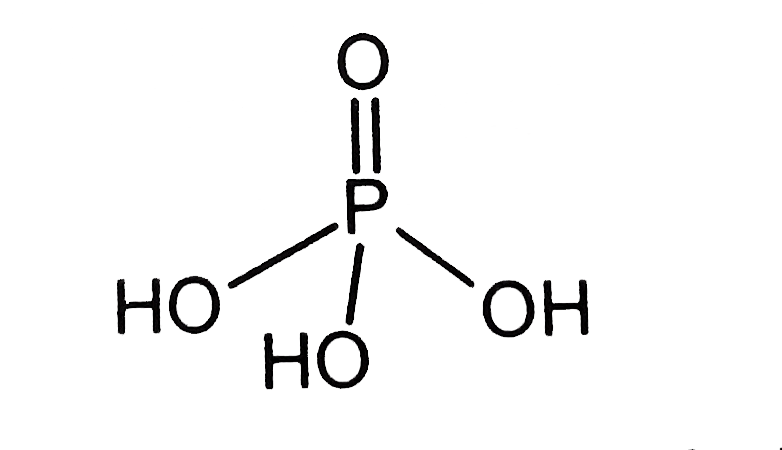
- What is the basicity of H(3)PO(4)?
Text Solution
|
- The uncertainty in the momentum of an electron is 2.0 × 10^(−5)kgms^(−...
Text Solution
|
- The uncertainty in the momentum of an electron is 3.0 × 10^(−5)kgms^(−...
Text Solution
|
- The uncertainty in the momentum of an electron is 5.0 × 10^(−5)kgms^(−...
Text Solution
|
- Bi(V) and Sb(V) which may be a stronger oxidizing agent and why ?
Text Solution
|
- Bi(2)O(3) is treated stronger oxidizing agent, write a balanced equati...
Text Solution
|
- The uncertainty in the momentum of an electron is 5.0 × 10^(−2)kgms^(−...
Text Solution
|
- The uncertainty in the momentum of an electron is 2.0 × 10^(−3)kgms^(−...
Text Solution
|
- The uncertainty in the momentum of an electron is 3.0 × 10^(−3)kgms^(−...
Text Solution
|
- Write balanced equation when NH(3) is dissolved in water.
Text Solution
|
- What is the oxidation number of phosphorus in H(3)PO(4) molecule ?
Text Solution
|
- What is the oxidation number of phosphorus in H(4)P(2)O(6) molecule ?
Text Solution
|
- What is the oxidation number of phosphorus in H(4)P(2)O(7) molecule ?
Text Solution
|
- What is the oxidation number of phosphorus in H(4)P(2)O(5) molecule ?
Text Solution
|
- What is the oxidation number of Carbon in Na(2)CO(3) molecule ?
Text Solution
|
- What is the oxidation number of Carbon in Na(2)C(2)O(4) molecule ?
Text Solution
|
- What is the oxidation number of Sulphur in Na(2)SO(3) molecule ?
Text Solution
|
- What is the oxidation number of Sulphur in Na(2)SO(4) molecule ?
Text Solution
|
- The oxidation number of nitrogen in NCl3 is
Text Solution
|
- The Oxidation number of S in (CH3)2SO is :
Text Solution
|