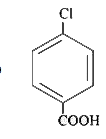
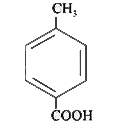
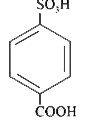
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST - 1 | JEE - 2020 -CHEMISTRY ( SECTION 2)
- Which of the following compounds is a weaker acid as compared to benzo...
Text Solution
|
- The enthalpy changes at 298 K in successive breaking of O-H bonds of ...
Text Solution
|
- To an acidified dichromate solution, a pinch of Na(2)O(2) is added an...
Text Solution
|
- The coagulation of 200 ml of a colloidal solution of gold is completel...
Text Solution
|
- The number of Oxygen atoms in one molecule of “Aspartame” is
Text Solution
|
- If pK(a)=-"log"K(a)=4, and K(a)=Cx^(2-) then Van't Hoff factor for wea...
Text Solution
|