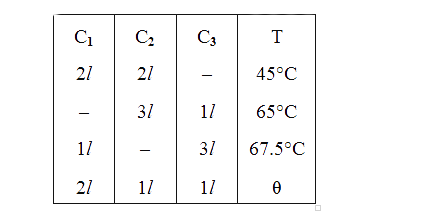Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-MOCK TEST 7-PHYSICS (SECTION 2)
- Three containers C1, C2 and C3 have water at different temperatures....
Text Solution
|
- A ball is dropped form the top of a 100 m high tower on a plant. In ...
Text Solution
|
- The frequency of one of the lines in Paschen series of hydrogen atom i...
Text Solution
|
- An asteroid is moving directly towards the centre of the earth. When a...
Text Solution
|
- The series combination of two batteries, both of the same emf 10 V, bu...
Text Solution
|