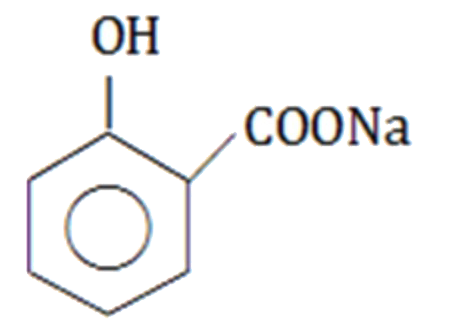
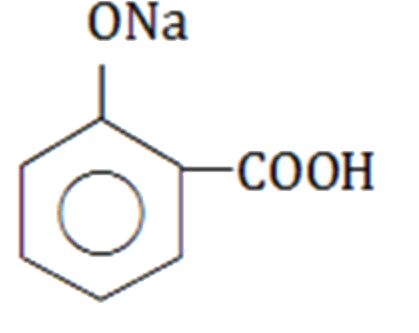
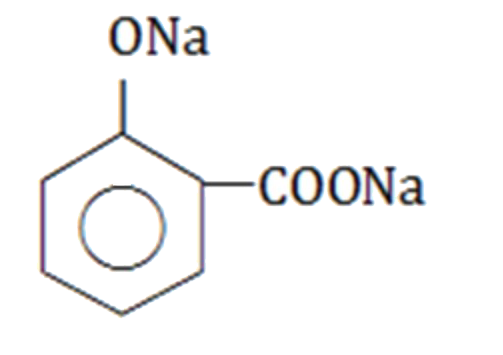
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Sodium bicarbonate reacts with salicylic acid to form
Text Solution
|
- Sodium bicarbocate reacts with salicylic acid to form
Text Solution
|
- Salixylic acid is prepared by reacting sodium phenoxide with carbon di...
Text Solution
|
- Sodium bicarbonate can react with:
Text Solution
|
- Sodium bicarbonate reacts with salicylic acid to form
Text Solution
|
- क्या होता है जब - सोडियम बाइकार्बोनेट तनु सल्फ्यूरिक अम्ल स...
Text Solution
|
- Phenol is an acid but does not react with sodium bicarbonate solution....
Text Solution
|
- फीनॉल एक अम्ल है परन्तु यह सोडियम बाइकार्बोनेट विलयन के साथ अभिक्रिया ...
Text Solution
|
- ఆమ్లాలు,కార్బొనేట్, బైకార్బొనేట్లతో చర్య జరిపి .. ఏర్పరుస్తాయి.
Text Solution
|