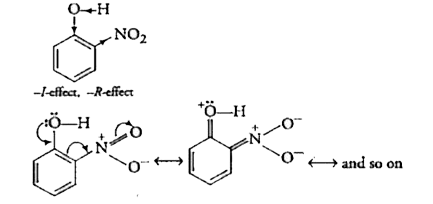
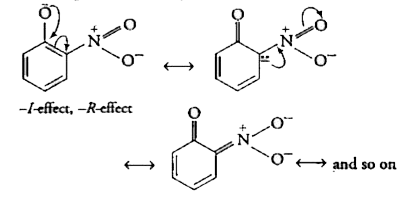
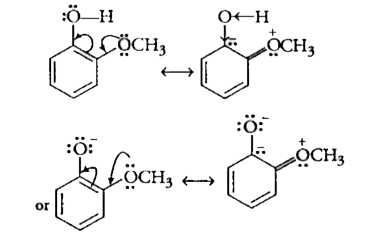
Text Solution
Verified by Experts
Topper's Solved these Questions
PHENOLS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (Long Answer Type Questions)|3 VideosPHENOLS
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS (A Multiple Choice Type Questions)|10 VideosPHENOLS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (Short Answer Type 1 Questions)|12 VideosHALOALKANES (ALKYL HALIDE)
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS)|5 VideosPOLYMERS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE ( LONG ANSWER TYPE QUESTIONS )|3 Videos
Similar Questions
Explore conceptually related problems