Text Solution
Verified by Experts
Topper's Solved these Questions
ARYL DIAZONIUM SALTS
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS (SHORT ANSWER TYPE II QUESTIONS) |1 VideosARYL DIAZONIUM SALTS
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS (LONG ANSWER TYPE QUESTIONS) |6 VideosARYL DIAZONIUM SALTS
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS (VERY SHORT ANSWER TYPE QUESTIONS) |4 VideosAMINES
ARIHANT PUBLICATION|Exercise Chapter Practice (Long Answer Type Questions)|2 VideosCARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |3 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-ARYL DIAZONIUM SALTS -ODISHA BUREAU.S TEXTBOOK SOLUTIONS (SHORT ANSWER TYPE I QUESTIONS)
- Suggest reason why excess mineral acid is used in diazo reaction.
Text Solution
|
- Complete the following equations. (i) C(6)H(5)N(2)Cl + H(2)O overse...
Text Solution
|
- Give the chemical equation for the following. (i) Diazotisation reac...
Text Solution
|
- How will you convert? (i) Aniline to nitrobenzene. (ii) Aniline to...
Text Solution
|
- How you will obtain p-hydroxyazobenzene from aniline?
Text Solution
|
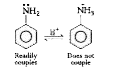
- What is coupling reaction ? Give an example.
Text Solution
|
- How will you carry out the conversion of aniline to m-bromonitrobenzen...
Text Solution
|
- Give the structure of A,B and C in the following reactions. (i) C(6...
Text Solution
|