Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS
BETTER CHOICE PUBLICATION|Exercise Question Bank (2 .4 Vapour Pressure of Liquid Solutions)|9 VideosSOLUTIONS
BETTER CHOICE PUBLICATION|Exercise Question Bank (2 .5 Ideal and non - ideal solutions)|11 VideosSOLUTIONS
BETTER CHOICE PUBLICATION|Exercise Question Bank (2 .2 Expressing Concentrations of Solutions)|12 VideosPOLYMERS
BETTER CHOICE PUBLICATION|Exercise QUESTIONS |42 VideosSTRUCTURE OF ATOM
BETTER CHOICE PUBLICATION|Exercise NUMERICAL PROBLEMS |6 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-SOLUTIONS-Question Bank (2 .3 Solubility)
- Define saturated and unsaturated solutions
Text Solution
|
- Define solubility. Name the factors on which solubility of a solute i...
Text Solution
|
- State Henry's law and mention its some important applications.
Text Solution
|
- What is the effect of temperature on solubility of a gas n a liquid ?
Text Solution
|
- Why do gases always tend to be less soluble in liquids as the temperat...
Text Solution
|
- What role does the molecular interaction play in a solution of alcohol...
Text Solution
|
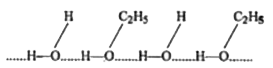
- Ethanol is an organic compound yet it is freely miscible with water.Ex...
Text Solution
|
- Carbon tetrachloride and water are immissible while ethyl alcohol an...
Text Solution
|