Rutherford.s `alpha`-scattering experiment. Ernest Rutherford was interested in knowing how the electrons are arranged within an atom.
Rutherford designed an experiment for this. In this experiment, fast moving alpha `(alpha)`-particles were made to fall on a thin gold foil.
(i) He selected a gold foil because he wanted as thin a layer as possible. This gold foil was about 1000 atoms thick.
(ii) `alpha`-particles are doubly-charged helium ions. Since they have a mass of 4u, the fast-moving `alpha`-particles have a considerable amount of energy.
(iii) It was expected that `alpha`-particles would be deflected by the sub-atomic particles in the gold atoms. Since the `alpha`-particles were much heavier than the protons. He did not expect to see large deflections.
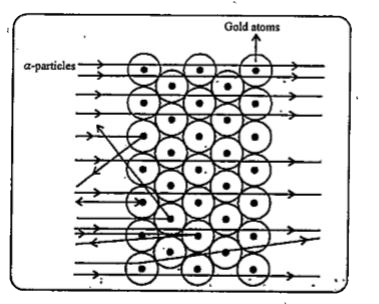
But, the `alpha`-particle scattering experiment gave totally unexpected results. The following observations were made :
(i) Most of the fast moving `alpha`-particles passed straight through the gold fall.
(ii) Some of the `alpha`-particles were deflected by the foil by small angles.
(iii) Surprisingly one out of every 12000 particles appeared to rebound.
In the words of Rutherford, "This result was almost as incredible as if you fire a 15-inch shell at a piece of tissue paper and it comes back and hits you".
Rutherford concluded from the `alpha`-particle scattering experiment that
(i) Most of the space inside the atom is empty because most of the `alpha`-particles passed through the gold foil without getting deflected.
(ii) very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
(iii) A very small fraction of `alpha`-particles were deflected by `180^(@)`, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.
From the data he also calculated that the radius of the nucleus is about `10^(5)` times less than the radius of the atom.
On the basis of his experiment, Rutherford put forward the nuclear model of an atom, which had the following features :
(i) There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
(ii) The electrons revolve around the nucleus in well-defined orbits.
(iii) The size of the nucleus is very small as compared to the size of the atom.
