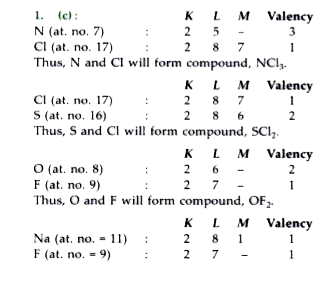
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG IIT JEE FOUNDATION-ATOMS AND MOLECULES -OLYMPIAD/HOTS CORNER
- A compound, PQ2 has the following arrangement of electrons : The ele...
Text Solution
|
- How many moles of electrons weigh one kilogram? (Mass of electron =9...
Text Solution
|
- A sample of MgCO3 contains 3.01 xx 10^(23) Mg^(2+) ions and 3.01 xx 1...
Text Solution
|
- What mass of hydrogen and oxygen will be produced on complete electrol...
Text Solution
|
- Ninhydrin having molecular formula C9H6O4, is commonly used by forensi...
Text Solution
|
- A sample of ascorbic acid (vitamin C) is synthesised in the laboratory...
Text Solution
|
- The total number of electrons present in 16 g of methane gas is:
Text Solution
|
- The number of atoms in 0.1 mol of a triatomic gas is:
Text Solution
|
- The number of particles present in one mole of any substance is equal ...
Text Solution
|
- Atoms are neither created nor destroyed in a chemical reaction. This p...
Text Solution
|
- Which of the following statements are correct? (Given : At. wt. of Ca ...
Text Solution
|
- Elements belonging to different groups of the periodic table are given...
Text Solution
|
- Which of the following contains maximum number of molecules?
Text Solution
|
- What is the mass of 12.044 xx 10^(23) number of O(2) molecules?
Text Solution
|
- If three gases X, Y and Z are arranged in increasing order of their re...
Text Solution
|
- The maximum number of molecules is present in :
Text Solution
|
- You are provided with 64 g of solid sulphur in container A and 64 g of...
Text Solution
|
- Arrange the following in the order of increasing mass (atomic mass: O...
Text Solution
|
- Valency of sulphur atom in SO(2) is:
Text Solution
|
- Some rocket engines use a mixture of hydrazine, N(2)H(4) and hydrogen...
Text Solution
|