But-2-ene is obtained as the major product. First n-butyl `(1^(@))` carbocation is formed . This being lesss stable rearranges to a more stable `2^(@)` carbocation which subsequently loses a proton to form but-2-ene in accordance with Saytzeff rule.
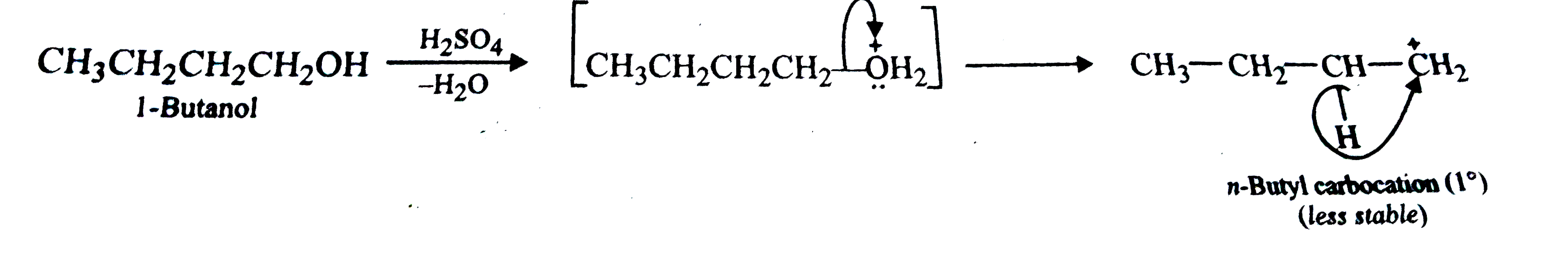
`underset(("More stable "))underset(2^(@) "Butyl carbocation ")(CH_(3)-CH_(2) -overset(+)(CH) -CH_(3)) overset(-H^(+))underset(("Saytzeff elimiN/Ation "))(to) underset("But -2-en (80 %)")(CH_(3)CH =CHCH_(3)) + underset("But -1-ene (20%)")(CH_(3) CH_(2) CH=CH_(2)`