Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
PRADEEP|Exercise Curiosity Question|5 VideosELECTROCHEMISTRY
PRADEEP|Exercise Problem for Practice|80 VideosELECTROCHEMISTRY
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|25 VideosD- AND F-BLOCK ELEMENTS
PRADEEP|Exercise IMPORTANT QUESTIONS|30 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
PRADEEP|Exercise Curiosity Questions|2 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ELECTROCHEMISTRY-Sample Problem
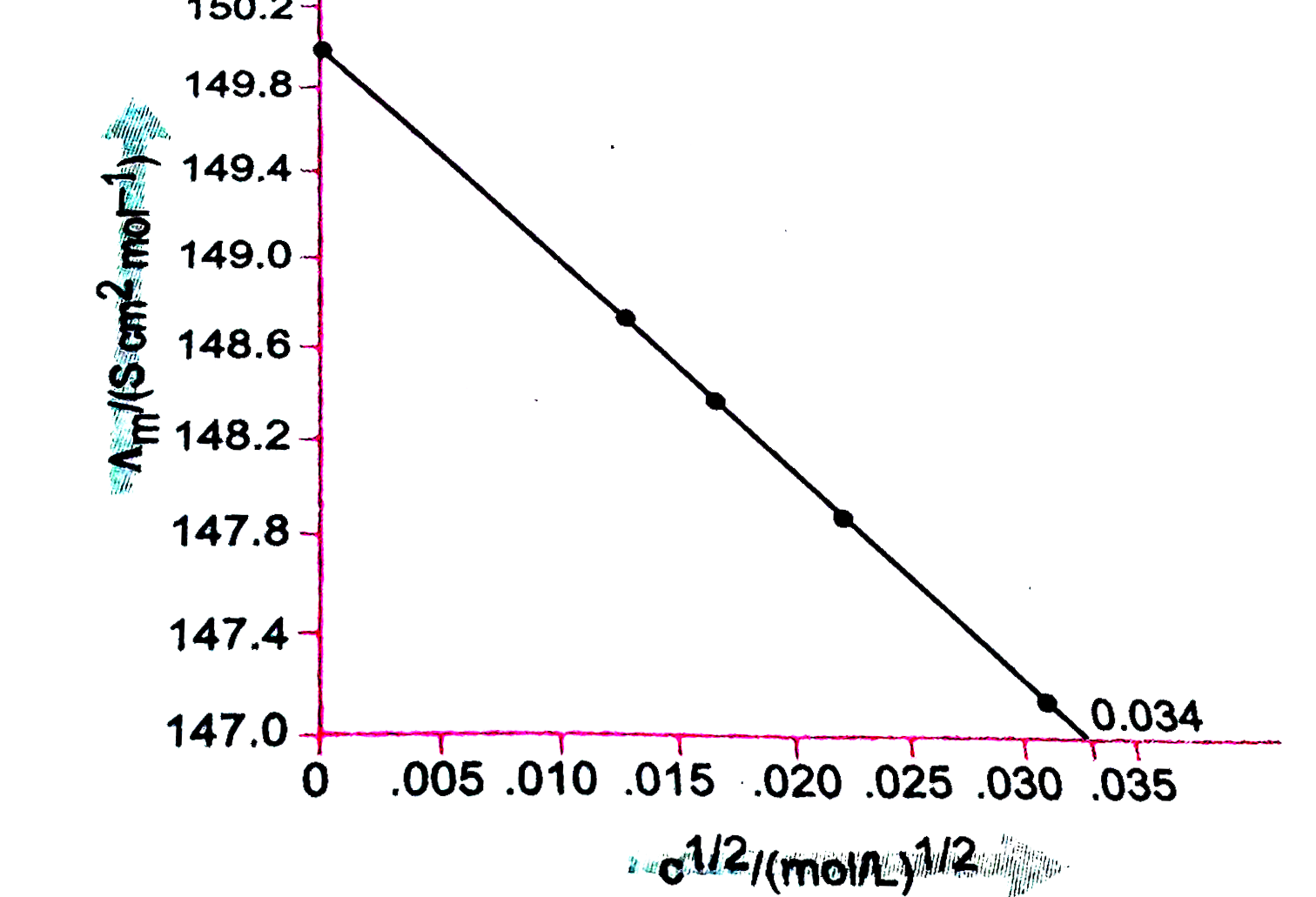
- The molar conductivity of KCl solution at different concentrations at ...
Text Solution
|
- Calculate the degree of dissociation (alpha) of acetic acid if its mol...
Text Solution
|
- The conductivity of a solution of AgCl at 298 K is found to be 1.382xx...
Text Solution
|
- The equilibrium constant for the cell Cu(s)+2Ag^+(aq)rarrCu^(2+)(aq)+2...
Text Solution
|
- If the molar conductivities at infinite dilution of NaCl, HCl and CH(3...
Text Solution
|
- wedge^(@).(m) for CaCl(2) and MgSO(4) from the given data. lambda(C...
Text Solution
|
- Molar conductivities at infinite dilution (at 298 K) of NH(4)CI, NaOH ...
Text Solution
|
- The conductivity of 0.001028 M acetic acid is 4.95xx10^(-5) S cm^(-1)....
Text Solution
|
- Calculate the standard EMF of a cell which invovles the following cell...
Text Solution
|
- A cell is prepared by dipping a copper rod in 1 M CuSO(4) solution and...
Text Solution
|
- Predict whether zinc and silver react with 1 M suphuric acid to give o...
Text Solution
|
- Can a solution of 1 M copper sulphate be stored in a vessel made of ni...
Text Solution
|
- Iodine (I(2)) and bromine (Br(2)) are added to a solution containing i...
Text Solution
|
- Calculate the EMF of the following concentration cell at 298K Zn|ZnS...
Text Solution
|
- A cell contains two hydrogen electrode. The negative electrode is in c...
Text Solution
|