Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- (a) Explain the steps for extraction of copper from its ore. Write the...
Text Solution
|
- Given below are the steps for extraction of copper from its ore. Write...
Text Solution
|
- (a) Write two equations to show the extraction of copper from its sulp...
Text Solution
|
- (a) Explain the steps for extraction of copper from its ore. Write the...
Text Solution
|
- (a) White the steps involved in the extraction of pure metals in th...
Text Solution
|
- Draw neat and labelled diagram of Besemer converter used in the extrac...
Text Solution
|
- How is copper metal extracted from its sulphide ore ?
Text Solution
|
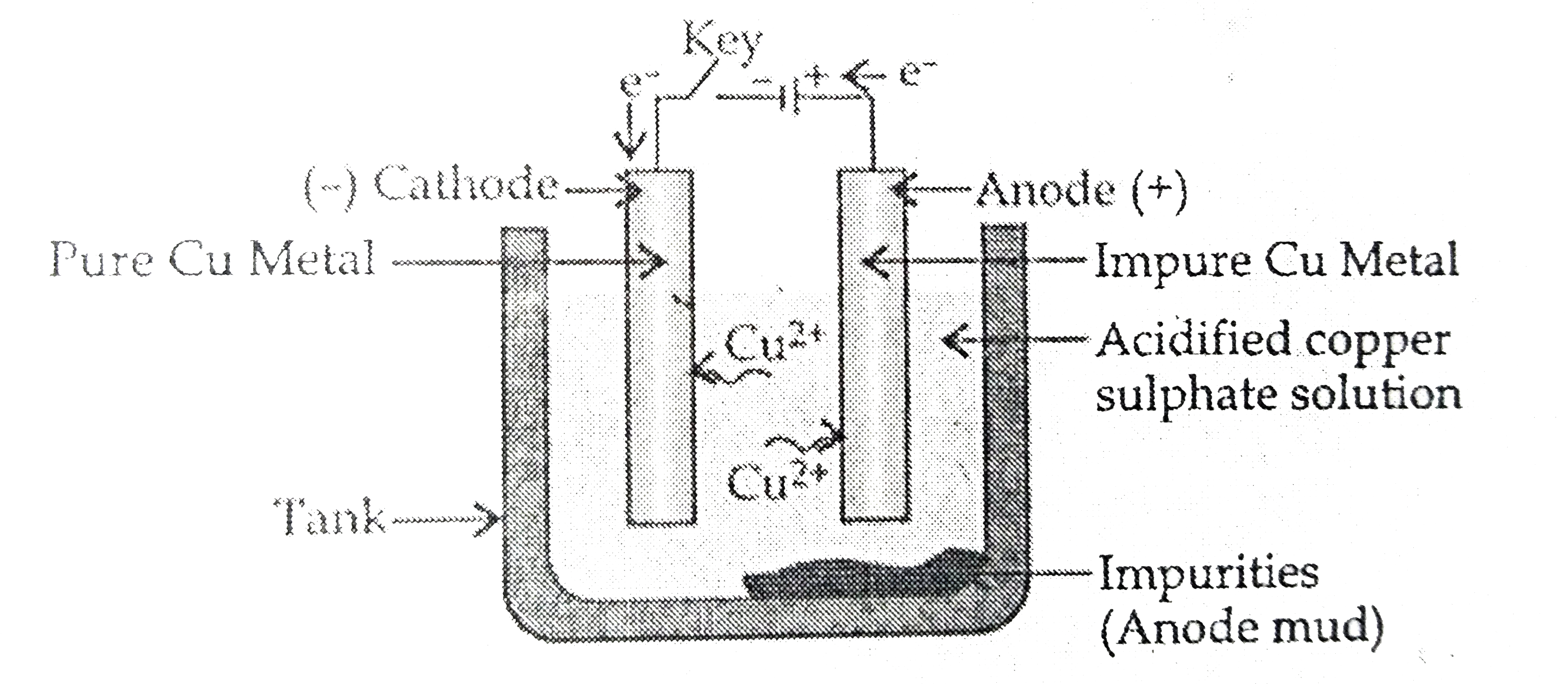
- (a) With the help of a labelled diagram, explain the process of elect...
Text Solution
|
- Explain the steps for extraction of copper from its sulphide ore. Writ...
Text Solution
|