(a) Limitations of Newlands' Law of Octaves :
(i) All the elements discovered at that time could not be classified into Octaves. The law was applicable only upto calcium, as after calcium every eighth element did not posses properties similar to that of the first.
(ii) Newland did not take into account the elements yet to be discovered. The newly discovered elements could not fit into his table.
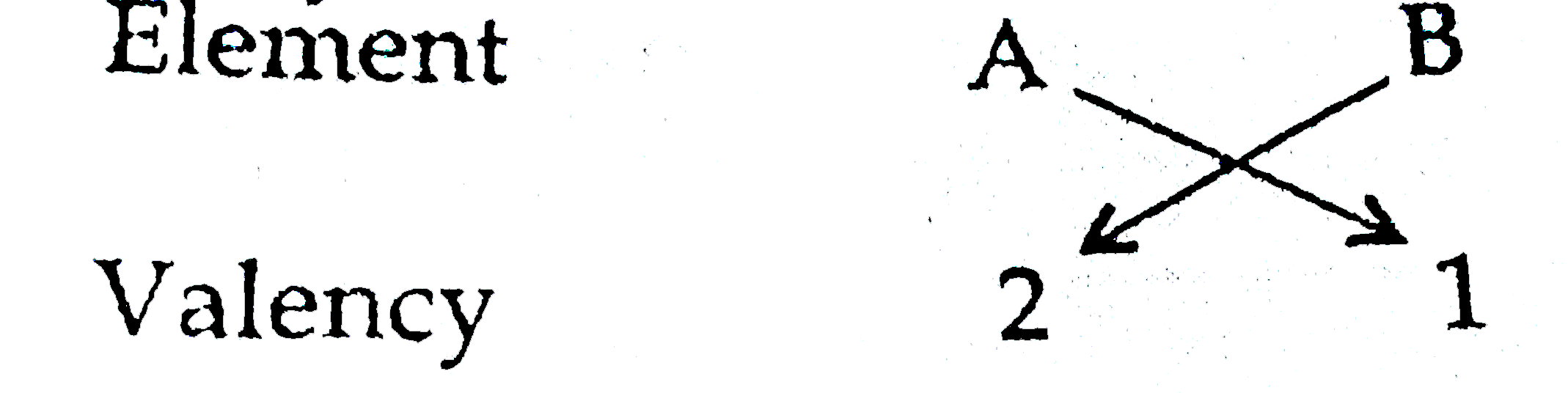
(b) Electronic configuration of elements 'A' and 'B' :
A(20 ) ` rArr ` 2, 8, 8, 2
B (17) ` rArr ` 2, 8, 7
Molecular formula of compound formed when element A reacts with elements B: `AB_(2)`
It is a neutral salt. It is a salt of a strong acid and a strong base.