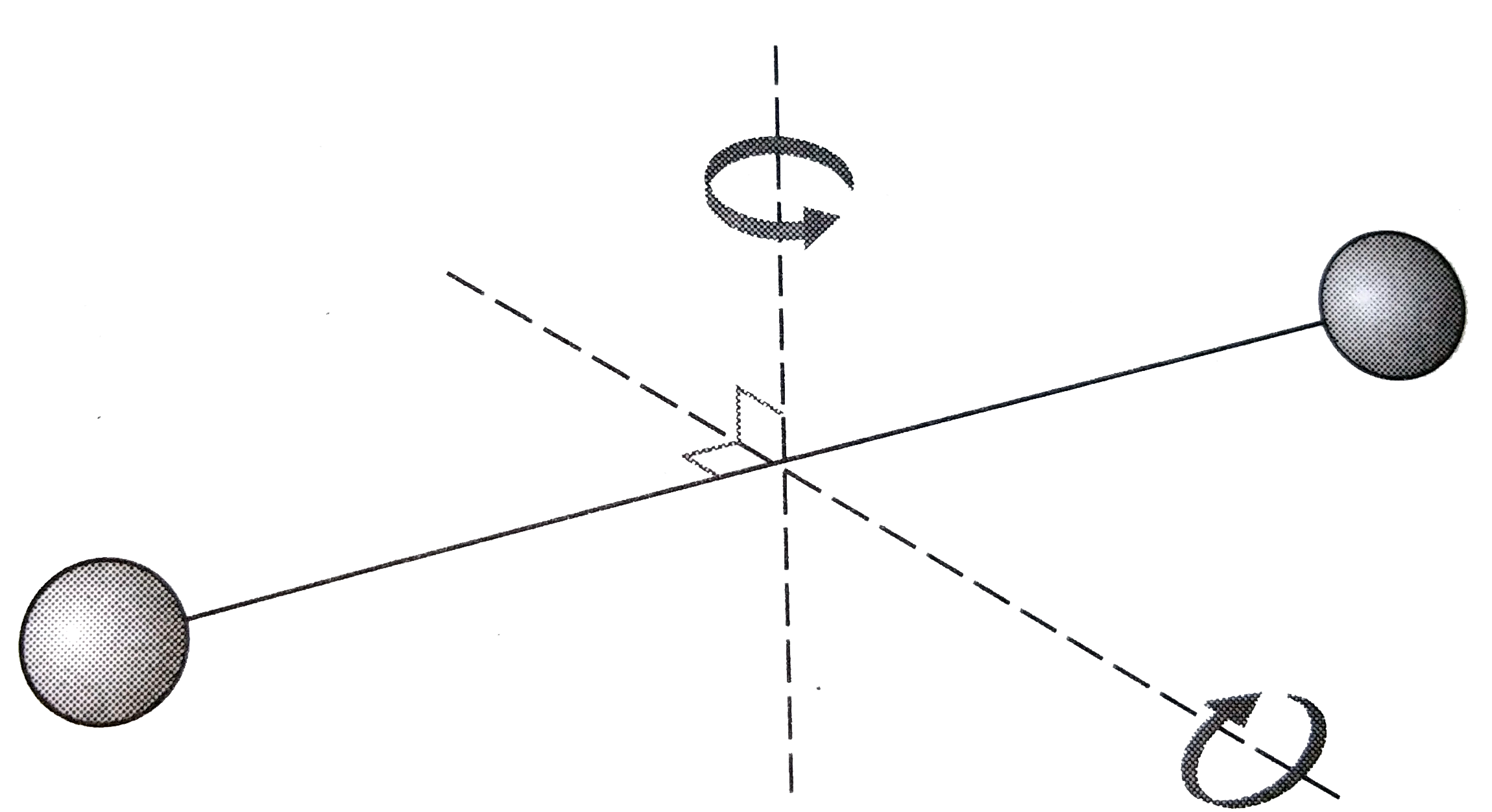
Text Solution
Verified by Experts
Topper's Solved these Questions
EXPLANATION, CHARACTERISTICS AND PROPERTIES
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Wave Theory of light|3 VideosEXPLANATION, CHARACTERISTICS AND PROPERTIES
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Interference and Diffraction|2 VideosEXPLANATION, CHARACTERISTICS AND PROPERTIES
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Stationary Waves|5 VideosEXPERIMENTS AND DIAGRAMS
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise ATOMS, MOLECULES AND NUCLEI|1 VideosINSTRUMENTS : CONSTRUCTION AND WORKING
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Semiconductors|6 Videos
Similar Questions
Explore conceptually related problems
NAVNEET PUBLICATION - MAHARASHTRA BOARD-EXPLANATION, CHARACTERISTICS AND PROPERTIES -Kinetic Theory of Gases and Radiation
- Explain the degrees of freedom for (i) An atom (ii) A diatomic mol...
Text Solution
|
- What is a heat engine? Explain the efficiency of a heat engine.
Text Solution
|
- Explain the blackbody radiation spectrum in terms of wavelength
Text Solution
|
- State any four characteristics of blackbody radiation spectra.
Text Solution
|