(A) Principle :
(1) The functioning of the fuel cell is based on the fact that the combustion reaction like `2H_(2(g)) + O_(2(g)) rarr 2H_(2)O_((g))` is exothermic redox reaction and can be used to produce electricity .
(2) The reactants of this fuel cell can be continuously supplied from outside, hence this type of galvanic cell can be used to supply electrical energy for a very long period.
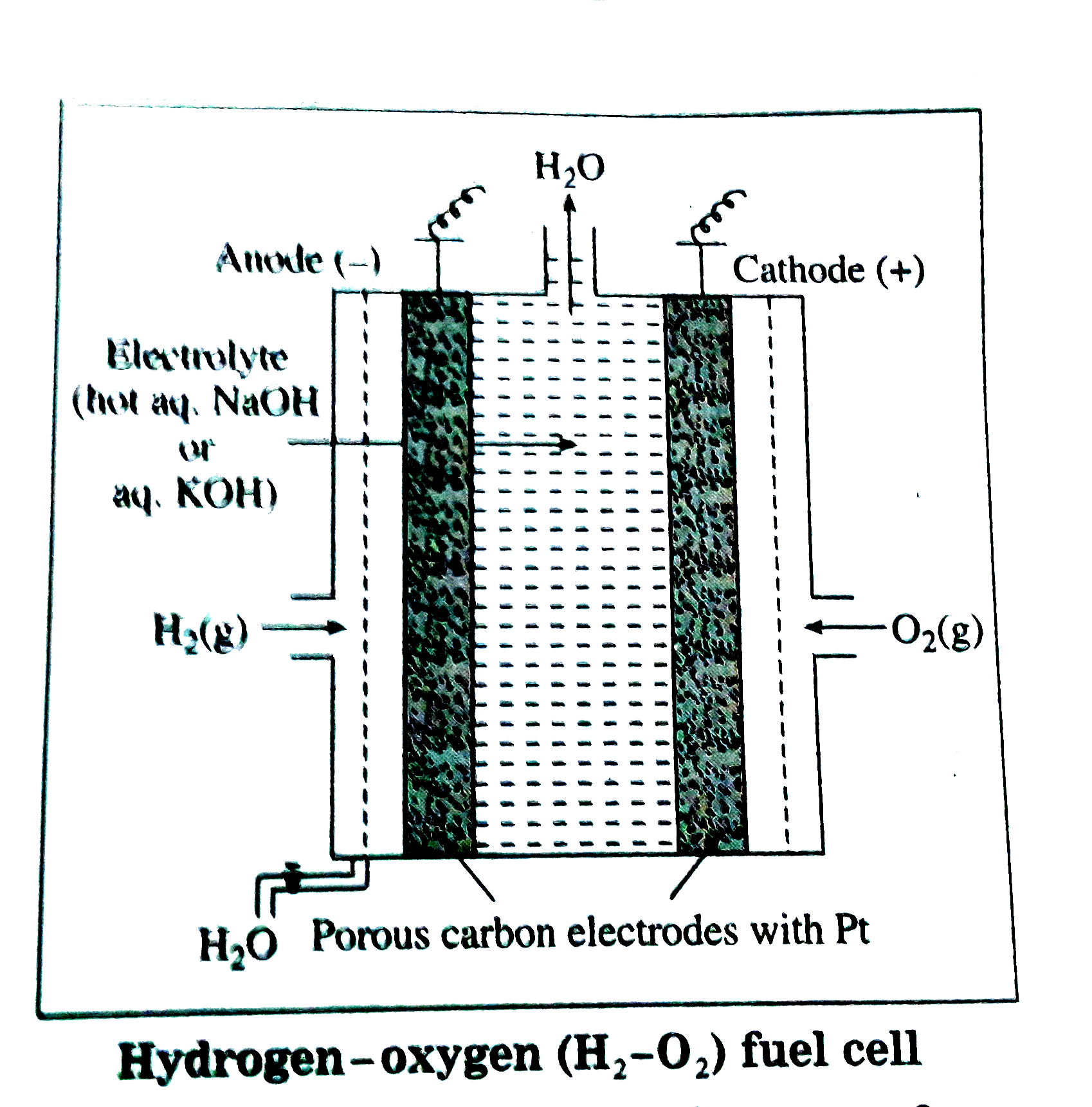
(B) Construction :
(1) The fuel cell has porous electrodes with suitable catalyst since combustion of hydrogen is a slow reaction.
(2) Anode and cathode cosists of porous carbon rods impregnated with finely divided platinum which acts as a catalyst for the reactions.
(3) The electrolyte used is not aqueous KOH solution in wich porous anode and cathode carbon rods are immersed.
(4) `H_(2)` is continuously bubbled through anode while `O_(2)` gas is bubbled through cathode.
(C ) Working (cell reactions) :
(1) Oxidation at anode :
`2H_(2(g)) + 4OH_((aq))^(-) rarr 4H_(2)O_((l)) + 4e^(-)` (oxidation half reaction)
(2) Reduction at cathode :
`O_(2(g)) + 2H_(2)O_((l)) + 4e^(-) rarr 4OH_((aq))^(-)` (reduction half reaction)
(3) Net cell reaction : Addition of both the above reactions at anode and cathode gives a net cell reaction.
`2H_(2(g)) + O_(2(g)) rarr 2H_(2)O_((l))` (overall cell reaction)
(D) Representation of the cell :
`""^(-)Pt|H_(2(g)) |underset((hot))(NaOH_((aq)))|O_(2(g))|Pt^(+)`
`E_("cell")^(0) = E_("cathode")^(0) - E_("anode")^(0)`
`= 0 .4- (-0.83)`
`= 1.23 V`
(E) Advantages :
(1) The cell reactions do not cause any pollution.
(2) The efficiency of this galvanic cell is the highest about `70%` as compared to ordinary galvanic cells.
(F) Drawbacks of `H_(2)l-O_(2)` fuel cell :
(1) The cell requires expensive electrodes like `Pt, Pd`.
(2) In practice, voltage is less than `1.23` volt due to spontaneous reactions at the electrodes.
(3) `H_(2)` gas is expensive and hazardous .
(G) Applications :
(1) It was successfully used in spacecraft.
(2) It has potential applications in automobiles power generators for domestic and industrial uses.
