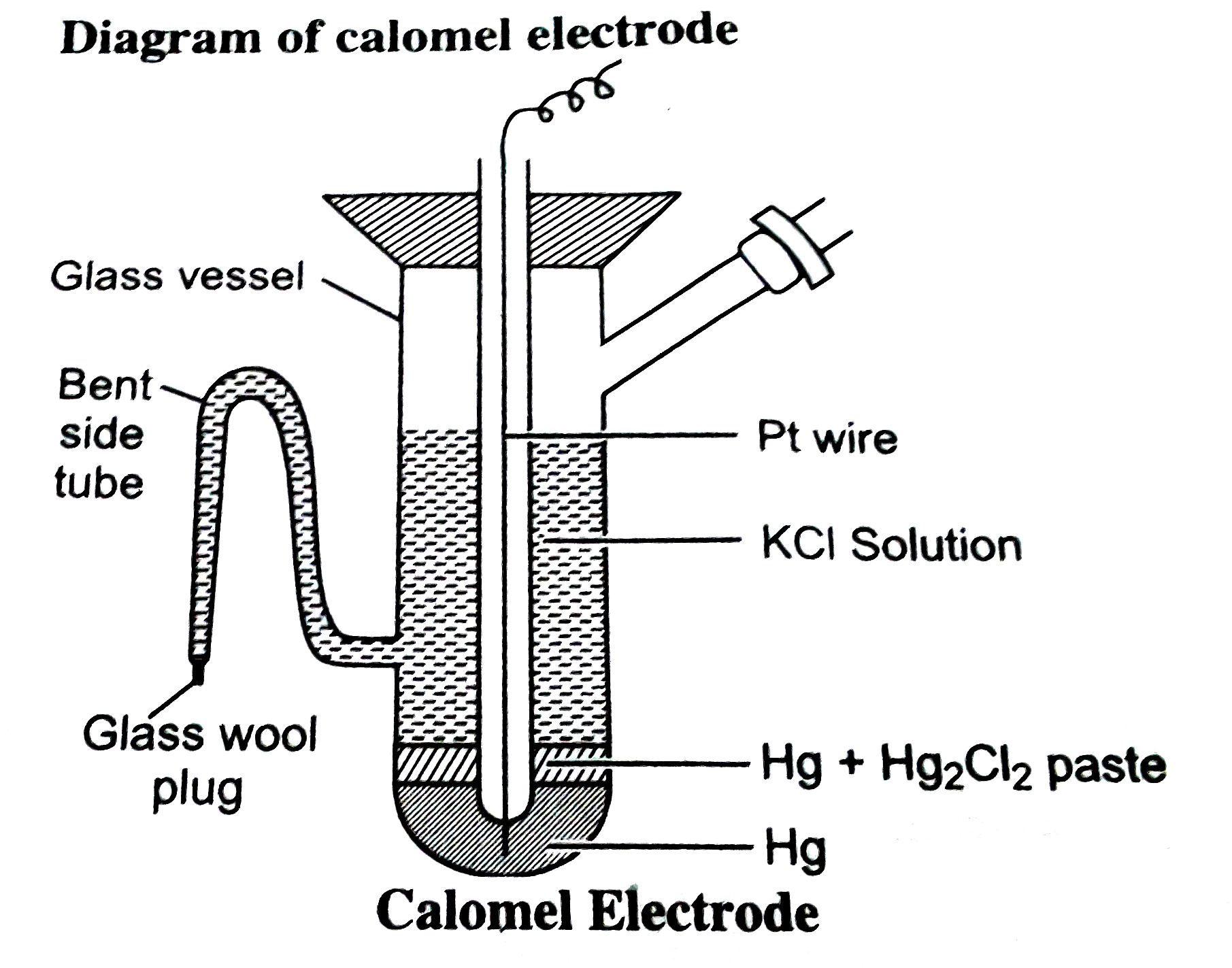
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GURUKUL PUBLICATION - MAHARASHTRA PREVIOUS YEAR PAPERS- MARCH 2014-Section - I
- What is 'boiling point' ? Drive a relation between DeltaH and DeltaU f...
Text Solution
|
- Write molecularity of the following reaction : 2NO((s))+O(2(g))to2NO...
Text Solution
|
- One mole of a gas expands by 3L against a constant pressure of 3 atmos...
Text Solution
|
- Calculate the amount of CaCl(2) (van't Hoff factor I =2*47) dissolved ...
Text Solution
|
- Describe anomalous behaviour of fluorine with the other elements of gr...
Text Solution
|
- Face centred cubic crystal lattice of copper has density of 8*966" g. ...
Text Solution
|
- What is the action of the following regents on ammonia : (a) Nessler...
Text Solution
|
- State the first and second law of electrolysis.
Text Solution
|
- Draw neat and labelled diagram of Besemer converter used in the extrac...
Text Solution
|
- Derive the relationship between half life and rate constant for first ...
Text Solution
|
- Derive the relation between DeltaG^(@) and equilibrium constant (K) ...
Text Solution
|
- Explain brown ring with the help of chemical equation.
Text Solution
|
- Explain, why do aquatic animals prefer to stay at lower leval of water...
Text Solution
|
- Crystalline solids and Amorphous solids.
Text Solution
|
- To get n-type doped semiconductor, impurity to be added to silicon sho...
Text Solution
|
- Number of faradays of electricity required to liberate 12 g of hydroge...
Text Solution
|
- What is molecular formula of oleum ?
Text Solution
|
- Purification of aluminium by electrolytic refining is carried out by :
Text Solution
|
- The rate of reaction of certain reaction is expressed as : (1)/(3)(d...
Text Solution
|
- A system absorbs 640 J heat and does work of 260 J, the change in inte...
Text Solution
|