Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GURUKUL PUBLICATION - MAHARASHTRA PREVIOUS YEAR PAPERS- JULY 2018-SECTION-II
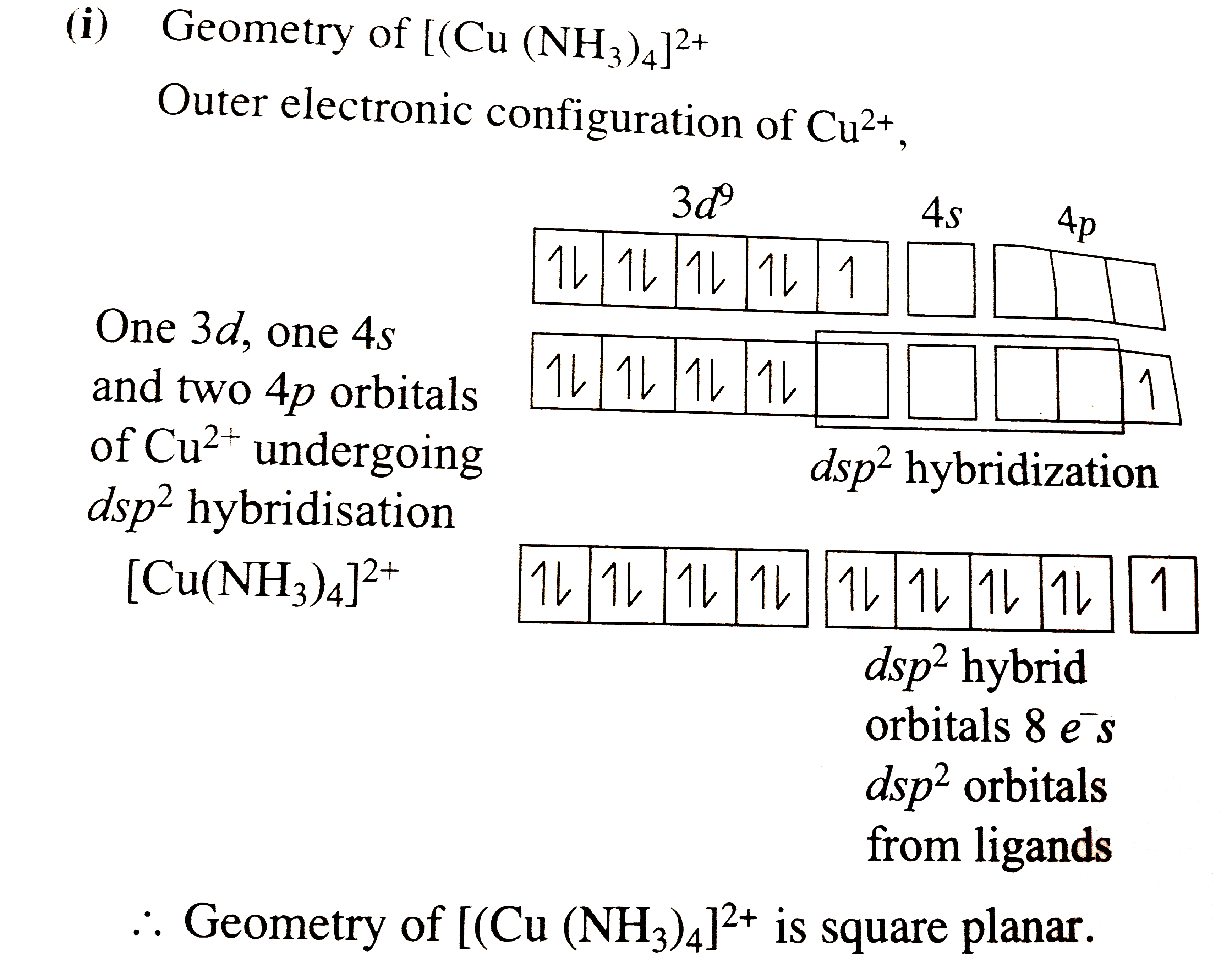
- Explain the geometry of [Cu(NH(3))(4)]^(2+) on the basis of hybridisa...
Text Solution
|
- What are 'd' and 'f' block elements ?
Text Solution
|
- Write a short note on Sandmeyer's reaction .
Text Solution
|
- What is the action of the following on isopropyl methyl ether ? (a) ...
Text Solution
|
- Write balanced equations for the following conversions : Cyclopropa...
Text Solution
|
- What is the action of lithium aluminium hydride in the presence of...
Text Solution
|
- How is glucose prepared from starch ?
Text Solution
|
- Define : (a) Analgesics (b) Antimicrobials
Text Solution
|
- Complete and rewrite the balanced chemical equation for the following...
Text Solution
|
- Write a preparation of phenol from cumene ? What happens when phenol...
Text Solution
|
- What are racemates ? What is the action of the following reagents o...
Text Solution
|
- What are elastomers ? Distinguish between thermoplastic polymers a...
Text Solution
|
- What is the action of the following on lanthanoids ? (a) water (...
Text Solution
|
- Draw the structures of Veronal and Thymine.
Text Solution
|
- Identify A and B from the following reaction and rewrite complete re...
Text Solution
|
- Write the preparation of benzoic acid from the following : (a) st...
Text Solution
|
- Write resonance structures of aniline. What is the action of benzene d...
Text Solution
|
- Write the formula for pentaammine chlorocobalt (III) sulphate.
Text Solution
|
- A polymer which contains ester linkage is :
Text Solution
|
- An antihistamine drug is :
Text Solution
|