Text Solution
Verified by Experts
Topper's Solved these Questions
REDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.1|10 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.2|18 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Assertion Reasoning Type|5 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY|Exercise Archives Subjective|8 Videos
Similar Questions
Explore conceptually related problems
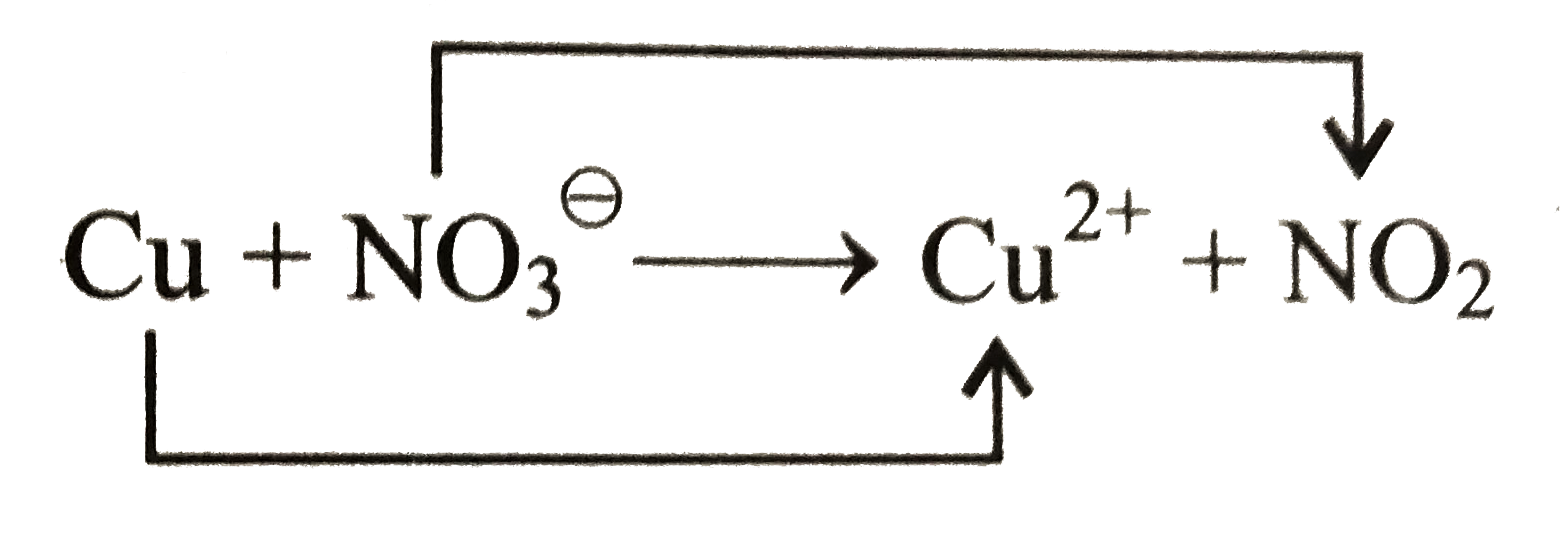
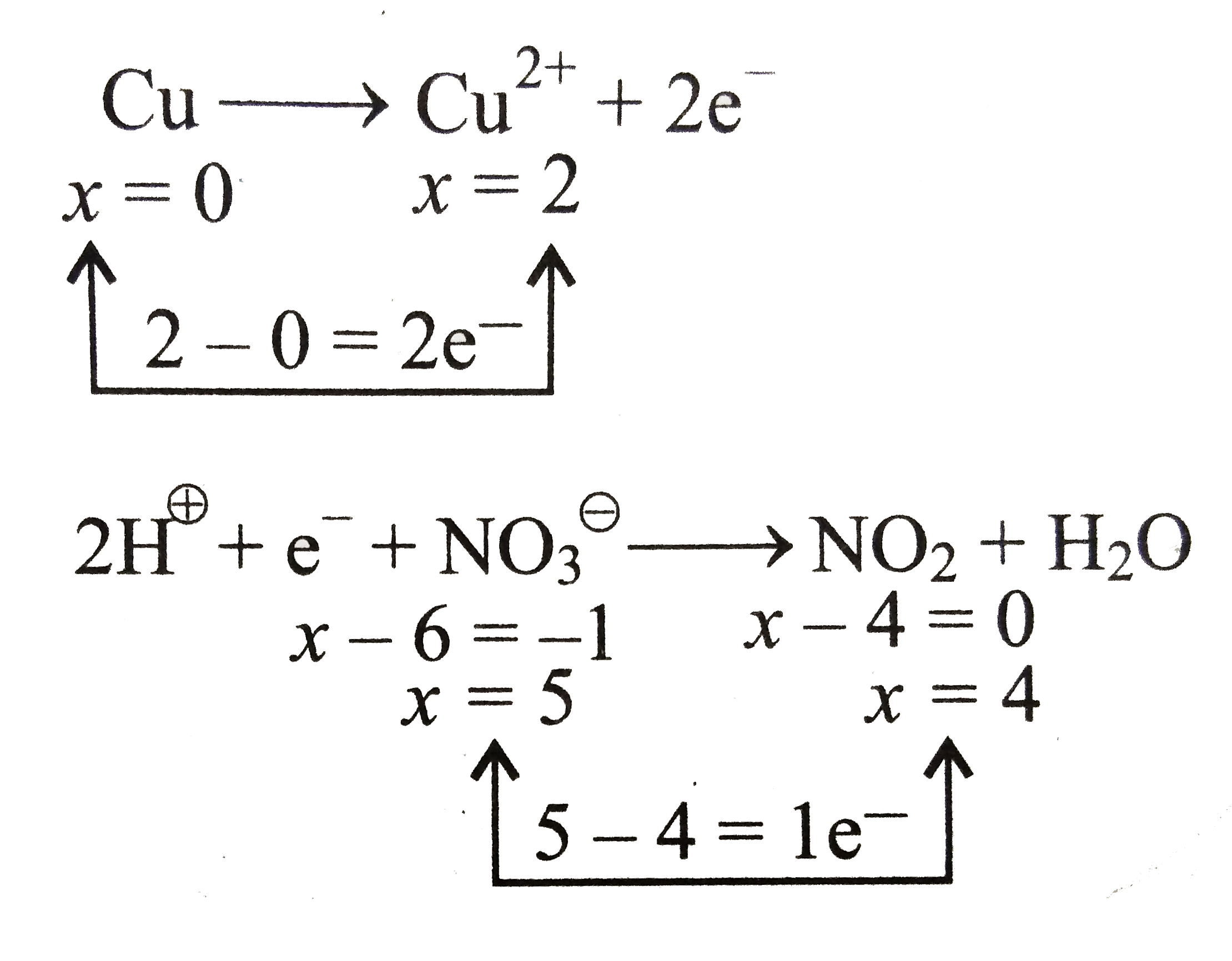 ….(i)
….(i)