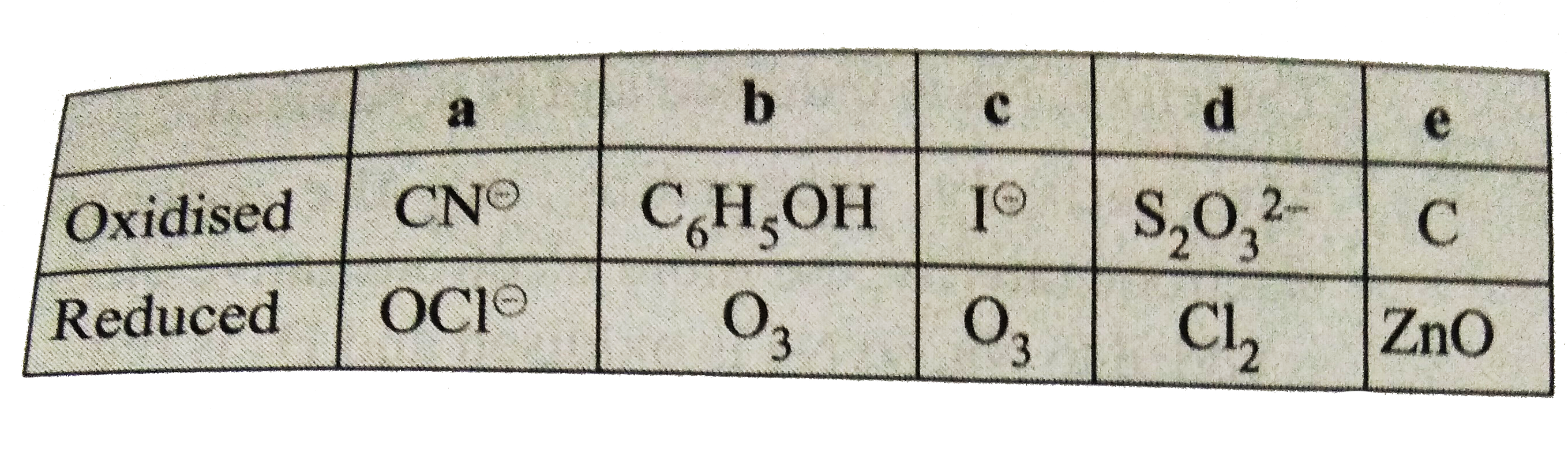
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercise|29 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Linked Comprehension)|24 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.1|10 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Assertion Reasoning Type|5 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY|Exercise Archives Subjective|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-REDOX REACTIONS-Ex 2.2
- Indicate the species which are oxidised and reduced in the following r...
Text Solution
|
- What is the oxidation stae of Cl in (a) CrO(2)Cl(2) , (b) HClO(4) ...
Text Solution
|
- Balance the following half-reactions in acidic medium: (a) IO(3)^(Ө)...
Text Solution
|
- Write balanced redox reactions for each of the following reactions: ...
Text Solution
|
- Balance the following chemical reactions (by ion electron method) (a...
Text Solution
|
- Write balanced ionoic half equation (oxidation and reduction) for each...
Text Solution
|
- Balance the following half reactions in basis medium: (a) CrO(4)^(2-...
Text Solution
|
- Write balanced net ionic equations for the following reactions in basi...
Text Solution
|
- Balanced the following equations: (a) H(2)O(2)+H^(o+)+Fe^(2+)rarr H(...
Text Solution
|
- For the redox reaction: Cr(2)O(7)^(2-)+H^(o+)+NirarrCr^(3+)+Ni^(2)+H...
Text Solution
|
- SO(2) under atomspheric condition changes to SO(x)^(2-). If oxidation ...
Text Solution
|
- Which of the following can act as an oxidising agent as well as a redu...
Text Solution
|
- Sulphur has highest oxidation state in
Text Solution
|
- The number of electrons involved in the reduction of nitrate (NO(3)^(ө...
Text Solution
|
- Oxidation number of P in Ba(H(2)PO(2))(2) is
Text Solution
|
- Which of the following is a disproportional reactions ?
Text Solution
|
- In balancing the half reaction CN^(ө)rarrCNO^(ө)(skeltan) The numb...
Text Solution
|
- Which of the following changes requires a reducing agent ?
Text Solution
|