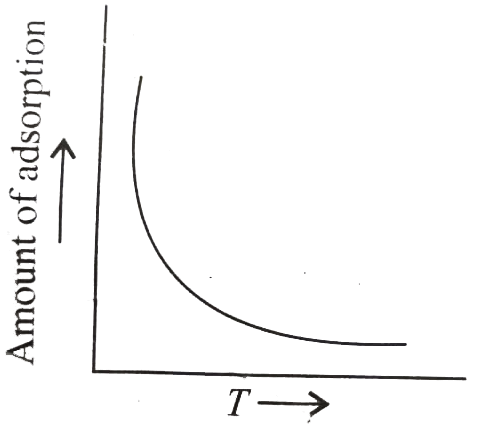
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Assertion-Reasoning|1 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Fill In The Blanks|1 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Multiple Correct|3 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.3 (Objective)|9 VideosSYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY|Exercise Exercises Assertion Reasoning|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SURFACE CHEMISTRY-Archives Single Correct
- The rate of physisorption increases with
Text Solution
|
- Lyophilic sols are
Text Solution
|
- Among the following, which surfactant will form micelles in aqueous so...
Text Solution
|
- Among the following electrolytes, which is the most effective coagulat...
Text Solution
|
- The coagulating power of electrolytes having inos Na^(o+),Al^(3+) and ...
Text Solution
|
- Methylene blue, from its aqueous solution, is adsorbed on activated ch...
Text Solution
|