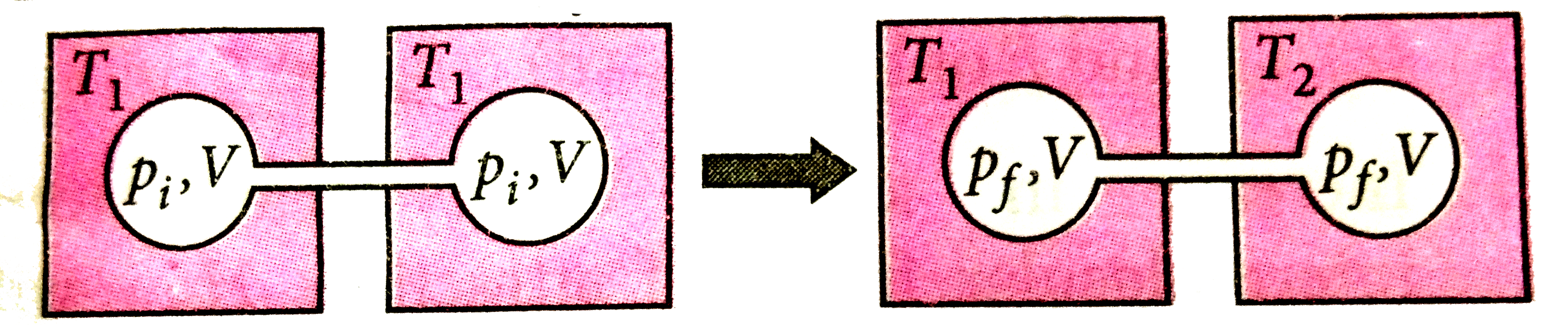
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
NCERT FINGERTIPS|Exercise Higher Order Thinking Skills|8 VideosSTATES OF MATTER
NCERT FINGERTIPS|Exercise NCERT Exemplar|10 VideosSTATES OF MATTER
NCERT FINGERTIPS|Exercise Liquid State|10 VideosSOME BASIC CONCEPTS OF CHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|10 VideosSTRUCTURE OF ATOM
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems