A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|12 VideosSOLID STATE
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise EXERCISE 1|77 VideosS-BLOCK ELEMENTS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise Exercise 2|20 VideosSOLUTIONS AND COLLIGATIVE PROPERTIES
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|25 Videos
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-SOLID STATE-EXERCISE 2
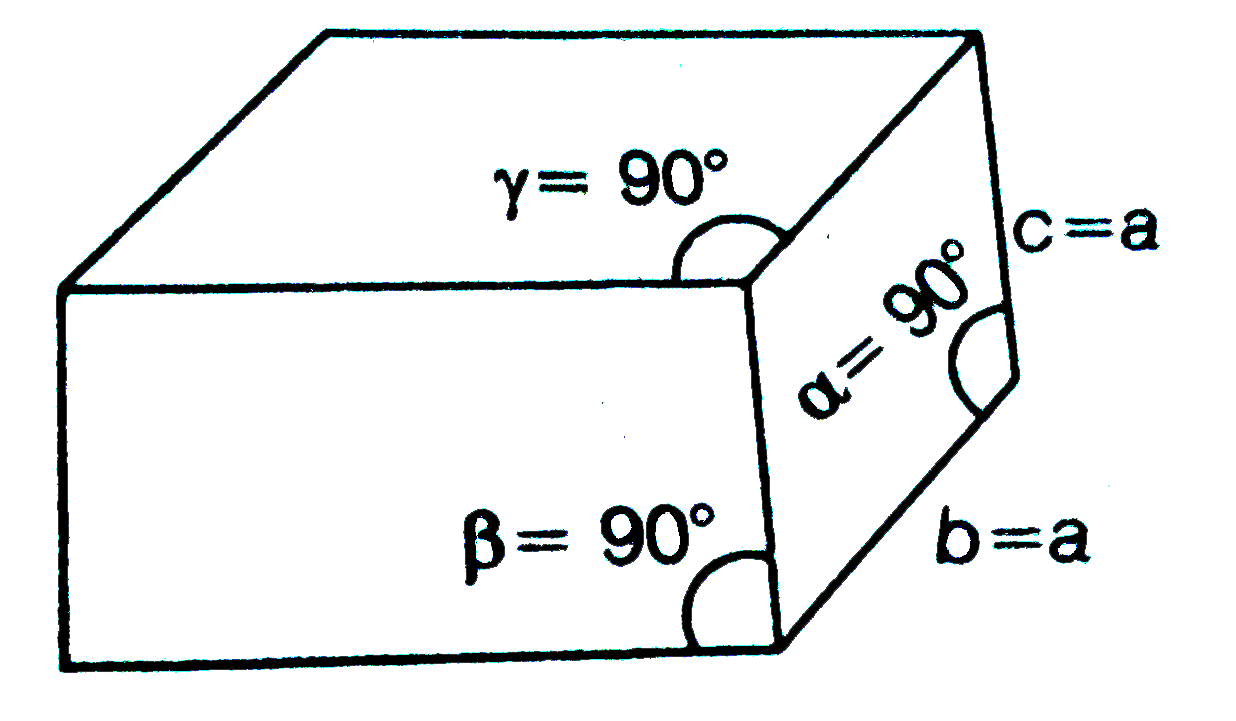
- Consider the figure , Which of the following system is exhibited...
Text Solution
|
- Consider the following statements I. InSb, AIP , GaAs are the compou...
Text Solution
|
- Analysis show that nickel oxide consists of nickel ion with 96% ions h...
Text Solution
|
- A solid compound contains X,Y and Z atoms in a cubic lattice with X at...
Text Solution
|
- In the distance between Na^(+) and CI^(-) ions in sodium chliride crys...
Text Solution
|
- The unit cell of a binary compound of A and B has ccp structure with A...
Text Solution
|
- The volume of atom present in a face-centred cubic unit cell of a meta...
Text Solution
|
- Ice crystallises in a hexagonal lattice . At the low temperature at wh...
Text Solution
|
- Calculate the ionic radius of Cs^+ ion assuming that the cll edge leng...
Text Solution
|
- Consider the following statements I. bcc structure has maximum pack...
Text Solution
|
- X-rays diffraction studies show that copper crystallizes in an fcc uni...
Text Solution
|
- A crystal made of metal crystallises into a lattice containing a seque...
Text Solution
|
- In which of the following crystals, alternate tetrahedral voids are oc...
Text Solution
|
- If the crystallises in zinc blende structure with I^- ions at lattice ...
Text Solution
|
- The packing efficiency of the two dimensional square unit cell shown b...
Text Solution
|
- Silver (atomic weight 108 g mol^(-1)) has a density of 10.5 g cm^(-3)....
Text Solution
|
- In orthorhombic , the value of a, b and c are respectively 4.2Å, 8.6 Å...
Text Solution
|
- Consider the following statements I. NaCl and KCl show metal excess ...
Text Solution
|
- Consider the following statements I. Fe, Co, Ni CrO2 are ferrimagnet...
Text Solution
|
- CsBr crystallizes in a body centred cubic lattice,The unit cell length...
Text Solution
|