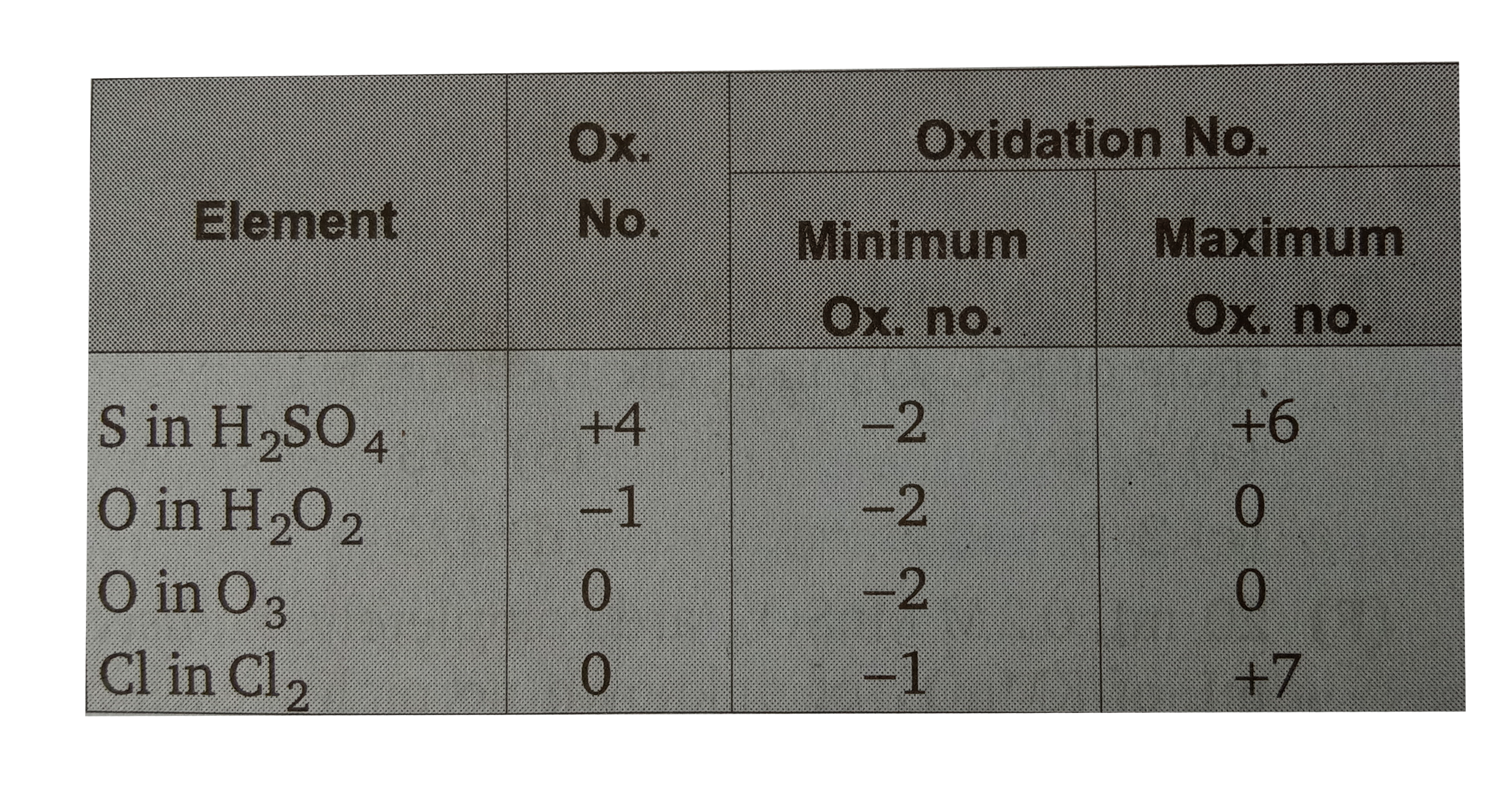
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
P BAHADUR|Exercise Exercise(8) Statement:Explanation type problems|19 VideosREDOX REACTIONS
P BAHADUR|Exercise Exercise (6) Integer answer type problems|18 VideosRADIO ACTIVITY
P BAHADUR|Exercise Exercies 9|99 VideosTHERMOCHEMISTRY
P BAHADUR|Exercise Exercise 9 Advance numerical problems|31 Videos
Similar Questions
Explore conceptually related problems
P BAHADUR-REDOX REACTIONS-Exercise (7) Comprehension based objective problems
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- Oxidation is de-electronation whereas reduction is electronation. Oxid...
Text Solution
|
- The equivalent weight of a species if acts as oxidant or reductant sho...
Text Solution
|
- The equivalent weight of a species if acts as oxidant or reductant sho...
Text Solution
|
- The equivalent weight of a species if acts as oxidant or reductant sho...
Text Solution
|
- The equivalent weight of a species if acts as oxidant or reductant sho...
Text Solution
|
- The number of moles of KMnO(4) required to oxidise 1 mol of Fe(C(2)O(4...
Text Solution
|
- The equivalent weight of a species if acts as oxidant or reductant sho...
Text Solution
|