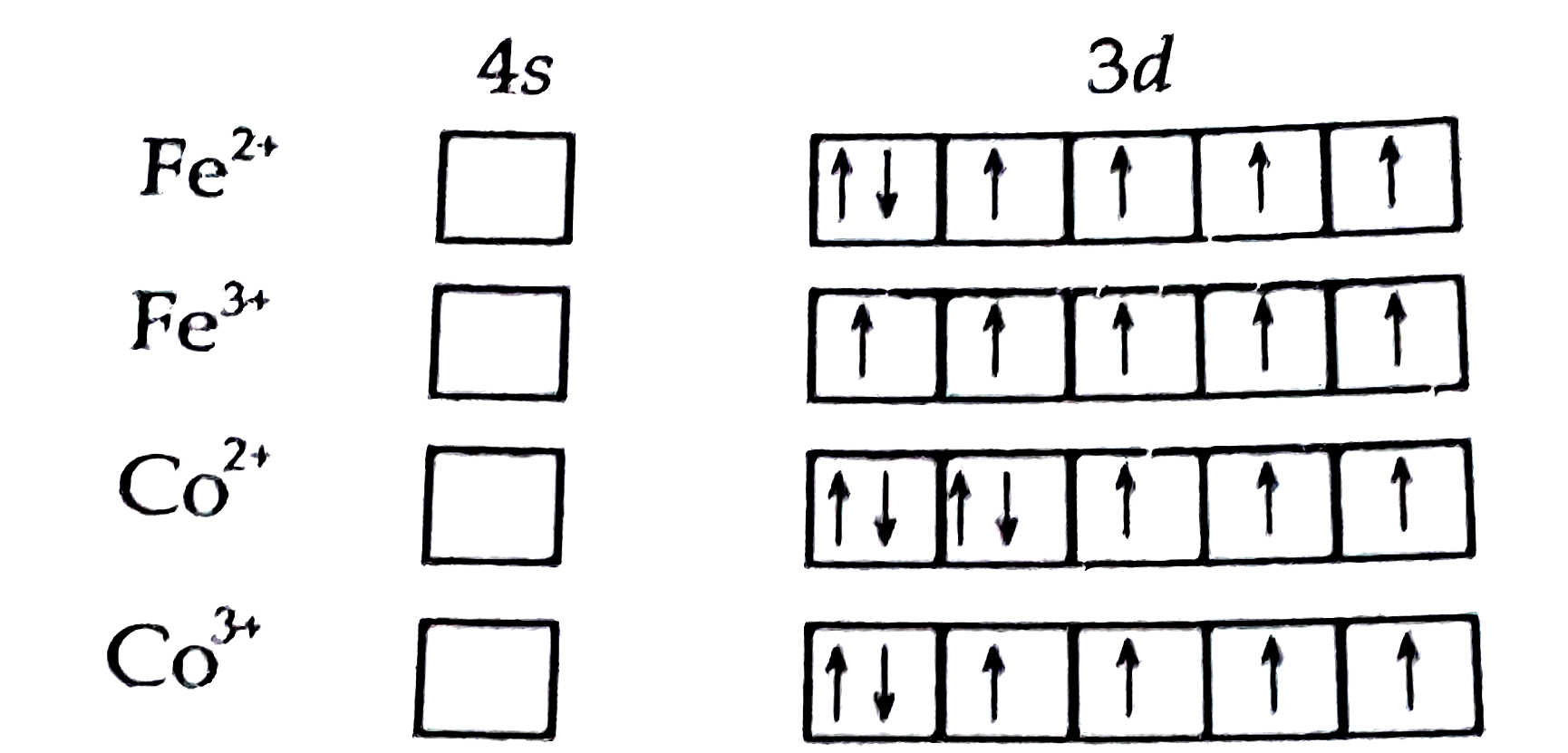
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-D-AND F-BLOCK ELEMENTS-MULTIPLE CHOICE QUESTIONS
- Which of the following has mximum number of unpaired electrons ?
Text Solution
|
- The number of incomplete shells in transition elements are
Text Solution
|
- The number of transition series in the periodic table are
Text Solution
|
- The first elements of first, second and third transition series respec...
Text Solution
|
- The lightest transition element is
Text Solution
|
- Which of the following has maximum I.E ?
Text Solution
|
- The electronic configuration of .(46)Pd is :
Text Solution
|
- First I.E. 5d elements are higher than those of 3d and 4d elements. Th...
Text Solution
|
- Pick out the incorrect statement for transition metals,
Text Solution
|
- Select incorrect statement (s)
Text Solution
|
- Pick out the incorrect statement for transition metals
Text Solution
|
- Magnetic moments 2.84 B.M is given by : (At. nos. ni = 28, Ti = 22, ...
Text Solution
|
- Which one of the following statements is not true?
Text Solution
|
- Non-stoichiometry is shown 1) due to variable valency of transition...
Text Solution
|
- Number of unpaired electrons increases in 3d series up to
Text Solution
|
- The first ionisation energies of the elements of the first transition ...
Text Solution
|
- Which one of the following ions has the highest megnetic moment ?
Text Solution
|
- Equivalent weight of KMnO(4) when it is convert into MnSO(4) is
Text Solution
|
- Wh ich of the following is weak reducing agent ?
Text Solution
|
- Which of the following has highest ionic radii
Text Solution
|