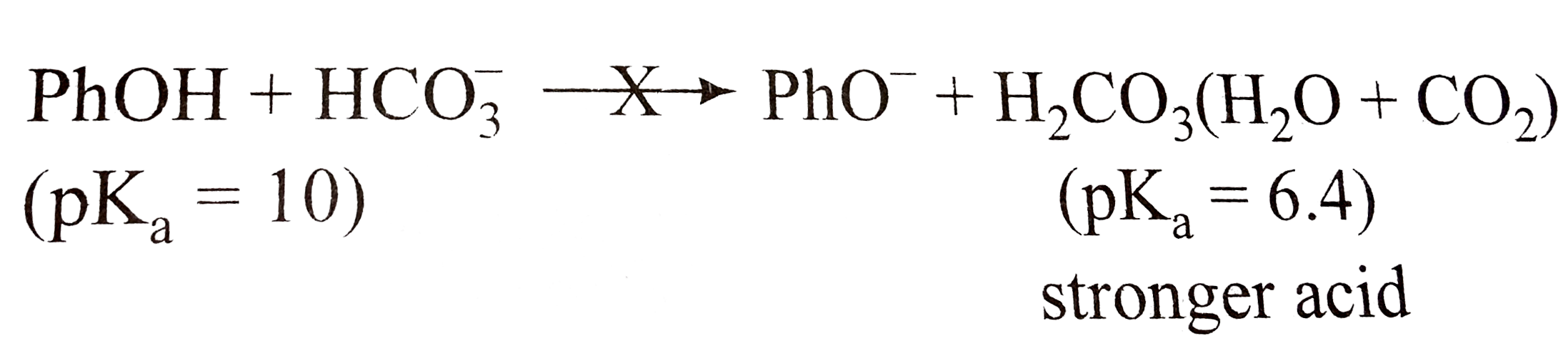Phenol is a weaker acid than carbonic acid, thus no forward reaction
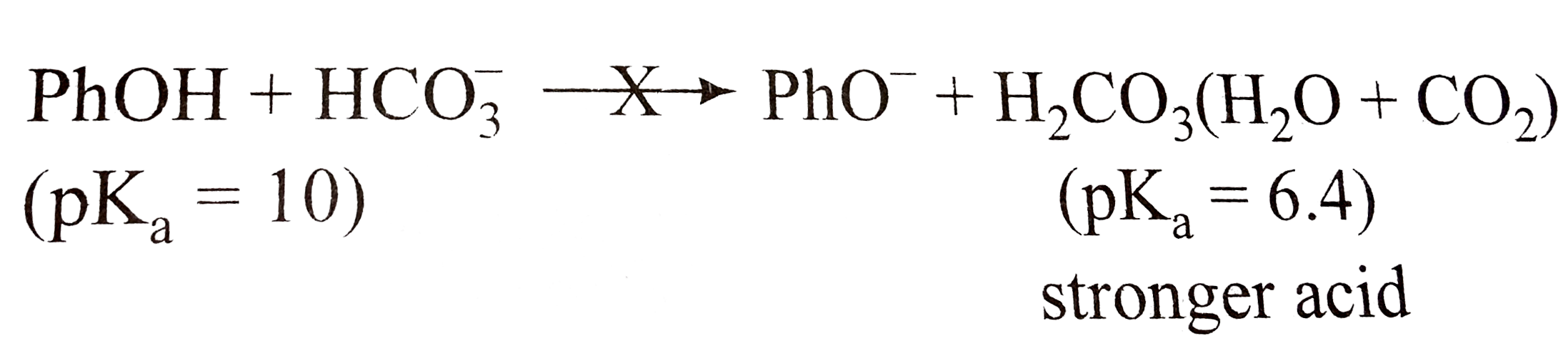
The equilibrium of Bronsted acid-base reactions favour the side with the weaker acid and base.Write the ionic equation as if it occurs and place the `pK_(a)` values under each acid. If the weaker acid is on the right side, the reaction occurs but if the weaker acid is on the left side, the reverse reaction occurs.
`underset(("stronger acid"))underset(pK_(a) = 5)(RCOOH) + HCO_(3)^(-) rarr RCOO^(-) + underset("weaker acid")underset(pK_(a) = 6.4)(H_(2)CO_(3)(H_(2)O + CO_(2))`
This reaction occurs
`{:(RCOOH+CO_(3)^(2-)rarr RCOO^(-)+,HCO_(3)^(-)),(pK_(a) = 5,pKa = 10.3),("stronger acid","weaker acid"):}`
This reaction occurs
`{:(PhOH+CO_(3)^(2-) hArr PhO^(-)+,HCO_(3)^(-)),(pK_(a)=10,pK_(a) = 10.3),("stronger acid","weaker acid"):}`
The last reaction above occurs. However, the result is borderline because any substituted phenol that has `pK_(a) gt 10.3` may not react.
(1) Unlike alcohols, phenols (stronger acids than water) trun blue litmus red.
(2) `C_(6)H_(5)OH + Na rarr C_(6)H_(5) bar(O)Na^(+) + (1)/(2) H_(2)`
(3) Because phanols are more acidic than water, following reaction goes essentially to completion and produces water - soluble sodium phenoxide.

The corresponding reaction of alcohol `(ROH)` with aqueous sodium hydroxide does not occur to a significant extent because alcohol is a weaker acid than water
`underset((pK_(a) = 18))underset("Weaker acid")(ROH +H_(2)O) underset(larr)overset(rarr): underset("Stronger base")(RbarONa^(+))+ underset((pKa_(a) = 16))underset("Stronger acid")(H_(2)O)`
The fact that phenols dissolve in aqueous sodium hydroxide while most alcohols with six carbon atoms or more do not, gives us a convenient means for distinguishing and separating phenols from most alcohols. (Alcohols with five carbon atoms ore fewer are quite soluble in water - some like methanol and ethanol are infinitely so-and so they dissolve in aqueous sodium hydroxide even through they are not converted to sodium alkoxides in appreciable amounts)