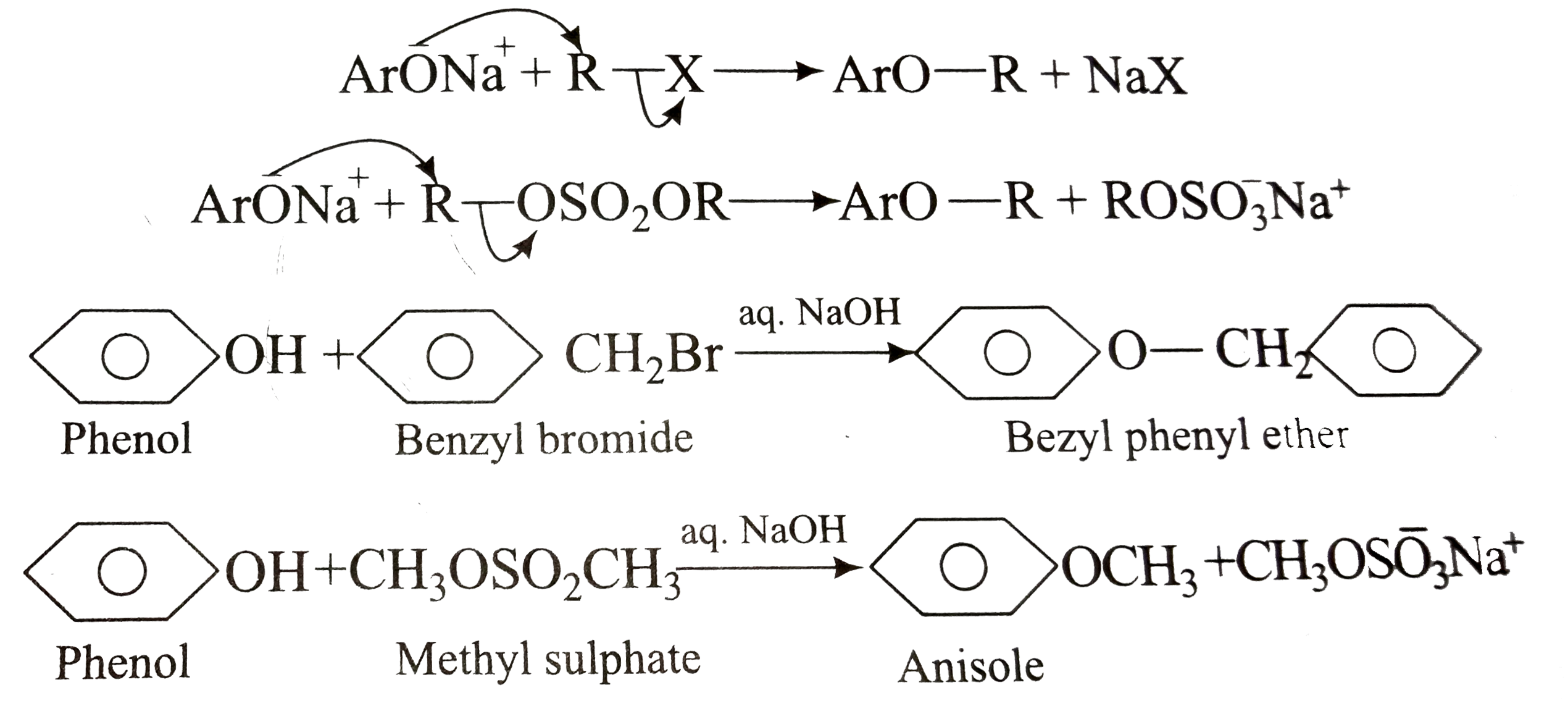
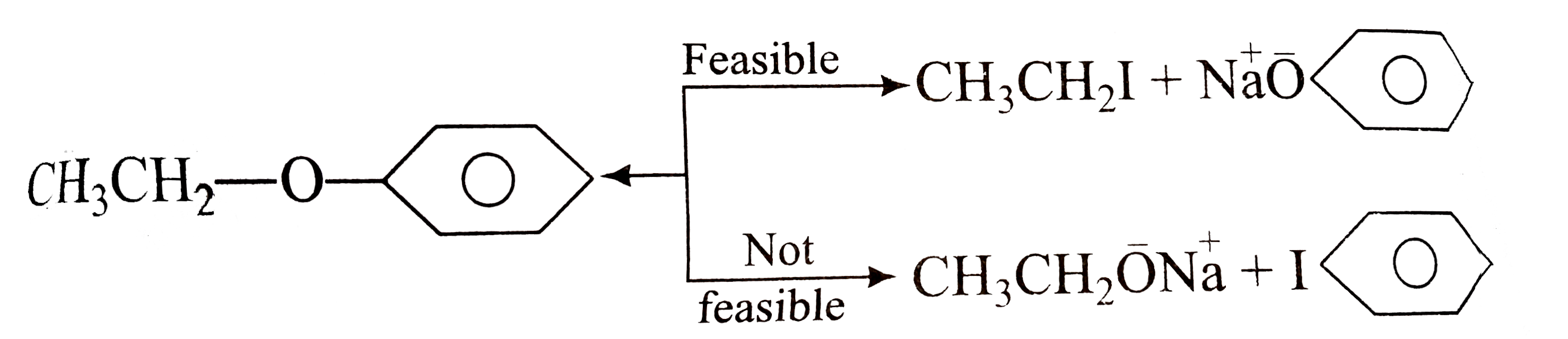
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-Follow -Up Test -10
- Which of the following is incorrect for phenol ?
Text Solution
|
- Which of the following is soluble in aqueous sodium bicarbonate ?
Text Solution
|
- Which of the following is the weakest acid?
Text Solution
|
- Which of the following is the strongest acid ?
Text Solution
|
- Which of the following is the weakest acid?
Text Solution
|
- Which of the following is the weakest acid?
Text Solution
|
- Which of the following is the weakest acid?
Text Solution
|
- Which of the following statements is incorrect ?
Text Solution
|
- Arrange the following compounds in order of decreasing acidic strength...
Text Solution
|
- In the reaction the product b is
Text Solution
|
- Phenolic ethers (alkaryl ethers, ArOR) are prepared by
Text Solution
|
- When phenol reacts with phosphorus pentachloride, the main product is
Text Solution
|